Glutamate in schizophrenia: a focused review and meta-analysis of ¹H-MRS studies
- PMID: 21746807
- PMCID: PMC3523901
- DOI: 10.1093/schbul/sbr069
Glutamate in schizophrenia: a focused review and meta-analysis of ¹H-MRS studies
Abstract
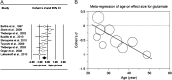
Schizophrenia is a severe chronic psychiatric illness, characterized by hallucinations and delusions. Decreased brain volumes have been observed in the disease, although the origin of these changes is unknown. Changes in the n-methyl-d-aspartate (NMDA)-receptor mediated glutamatergic neurotransmission are implicated, since it is hypothesized that NMDA-receptor dysfunction in schizophrenia leads to increased glutamate release, which can have excitotoxic effects. However, the magnitude and extent of changes in glutamatergic metabolites in schizophrenia are not clear. With (1)H magnetic resonance spectroscopy ((1)H-MRS), in vivo information about glutamate and glutamine concentrations can be obtained in the brain. A systematic search through the MEDLINE database was conducted to identify relevant (1)H-MRS studies that examined differences in glutamate and glutamine concentrations between patients with schizophrenia and healthy control subjects. Twenty-eight studies were identified and included a total of 647 patients with schizophrenia and 608 healthy-control subjects. For each study, Cohen's d was calculated and main effects for group analyses were performed using the random-effects model. Medial frontal region glutamate was decreased and glutamine was increased in patients with schizophrenia as compared with healthy individuals. Group-by-age associations revealed that in patients with schizophrenia, glutamate and glutamine concentrations decreased at a faster rate with age as compared with healthy controls. This could reflect aberrant processes in schizophrenia, such as altered synaptic activity, changed glutamate receptor functioning, abnormal glutamine-glutamate cycling, or dysfunctional glutamate transport.
Figures


References
-
- Wright IC, Rabe-Hesketh S, Woodruff PWR, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. - PubMed
-
- Van Haren NEM, Hulshoff Pol HE, Schnack HG, et al. Progressive brain volume loss in schizophrenia over the course of the illness: evidence of maturational abnormalities in early adulthood. Biol Psychiatry. 2008;63:106–113. - PubMed
-
- Cahn W, Hulshoff Pol HE, Lems EBTE, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59:1002–1010. - PubMed
-
- Borgwardt SJ, McGuire PK, Aston J, et al. Reductions in frontal, temporal and parietal volume associated with the onset of psychosis. Schizophr Res. 2008;106:108–114. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

