Synthesis of hydroxylated sterols in transgenic Arabidopsis plants alters growth and steroid metabolism
- PMID: 21746809
- PMCID: PMC3165889
- DOI: 10.1104/pp.110.171199
Synthesis of hydroxylated sterols in transgenic Arabidopsis plants alters growth and steroid metabolism
Abstract
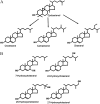
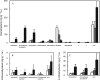
To explore mechanisms in plant sterol homeostasis, we have here increased the turnover of sterols in Arabidopsis (Arabidopsis thaliana) and potato (Solanum tuberosum) plants by overexpressing four mouse cDNA encoding cholesterol hydroxylases (CHs), hydroxylating cholesterol at the C-7, C-24, C-25, or C-27 positions. Compared to the wild type, the four types of Arabidopsis transformant showed varying degrees of phenotypic alteration, the strongest one being in CH25 lines, which were dark-green dwarfs resembling brassinosteroid-related mutants. Gas chromatography-mass spectrometry analysis of extracts from wild-type Arabidopsis plants revealed trace levels of α and β forms of 7-hydroxycholesterol, 7-hydroxycampesterol, and 7-hydroxysitosterol. The expected hydroxycholesterol metabolites in CH7-, CH24-, and CH25 transformants were identified and quantified using gas chromatography-mass spectrometry. Additional hydroxysterol forms were also observed, particularly in CH25 plants. In CH24 and CH25 lines, but not in CH7 ones, the presence of hydroxysterols was correlated with a considerable alteration of the sterol profile and an increased sterol methyltransferase activity in microsomes. Moreover, CH25 lines contained clearly reduced levels of brassinosteroids, and displayed an enhanced drought tolerance. Equivalent transformations of potato plants with the CH25 construct increased hydroxysterol levels, but without the concomitant alteration of growth and sterol profiles observed in Arabidopsis. The results suggest that an increased hydroxylation of cholesterol and/or other sterols in Arabidopsis triggers compensatory processes, acting to maintain sterols at adequate levels.
Figures





References
-
- Bergenstråhle A, Tillberg E, Jonsson L. (1993) Effects of ethephon and norbornadiene on sterol and glycoalkaloid biosynthesis in potato tuber discs. Physiol Plant 89: 301–308
-
- Cao S, Xu Q, Cao Y, Qian K, An K, Zhu Y, Binzeng H, Zhao H, Kuai B. (2005) Loss-of-function mutations in DET2 gene lead to an enhanced resistance to oxidative stress in Arabidopsis. Physiol Plant 123: 57–66
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

