Spatial organization of RYRs and BK channels underlying the activation of STOCs by Ca(2+) sparks in airway myocytes
- PMID: 21746845
- PMCID: PMC3149436
- DOI: 10.1085/jgp.201110626
Spatial organization of RYRs and BK channels underlying the activation of STOCs by Ca(2+) sparks in airway myocytes
Abstract
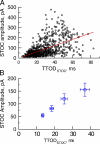
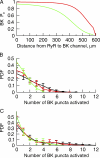
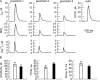
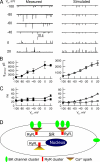
Short-lived, localized Ca(2+) events mediate Ca(2+) signaling with high efficiency and great fidelity largely as a result of the close proximity between Ca(2+)-permeable ion channels and their molecular targets. However, in most cases, direct evidence of the spatial relationship between these two types of molecules is lacking, and, thus, mechanistic understanding of local Ca(2+) signaling is incomplete. In this study, we use an integrated approach to tackling this issue on a prototypical local Ca(2+) signaling system composed of Ca(2+) sparks resulting from the opening of ryanodine receptors (RYRs) and spontaneous transient outward currents (STOCs) caused by the opening of Ca(2+)-activated K(+) (BK) channels in airway smooth muscle. Biophysical analyses of STOCs and Ca(2+) sparks acquired at 333 Hz demonstrate that these two events are associated closely in time, and approximately eight RYRs open to give rise to a Ca(2+) spark, which activates ∼15 BK channels to generate a STOC at 0 mV. Dual immunocytochemistry and 3-D deconvolution at high spatial resolution reveal that both RYRs and BK channels form clusters and RYR1 and RYR2 (but not RYR3) localize near the membrane. Using the spatial relationship between RYRs and BK channels, the spatial-temporal profile of [Ca(2+)] resulting from Ca(2+) sparks, and the kinetic model of BK channels, we estimate that an average Ca(2+) spark caused by the opening of a cluster of RYR1 or RYR2 acts on BK channels from two to three clusters that are randomly distributed within an ∼600-nm radius of RYRs. With this spatial organization of RYRs and BK channels, we are able to model BK channel currents with the same salient features as those observed in STOCs across a range of physiological membrane potentials. Thus, this study provides a mechanistic understanding of the activation of STOCs by Ca(2+) sparks using explicit knowledge of the spatial relationship between RYRs (the Ca(2+) source) and BK channels (the Ca(2+) target).
Figures


References
-
- Bao R., Lifshitz L.M., Tuft R.A., Bellvé K., Fogarty K.E., ZhuGe R. 2008. A close association of RyRs with highly dense clusters of Ca2+-activated Cl− channels underlies the activation of STICs by Ca2+ sparks in mouse airway smooth muscle. J. Gen. Physiol. 132:145–160 10.1085/jgp.200709933 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

