Alignment of transmembrane regions in the cystic fibrosis transmembrane conductance regulator chloride channel pore
- PMID: 21746847
- PMCID: PMC3149817
- DOI: 10.1085/jgp.201110605
Alignment of transmembrane regions in the cystic fibrosis transmembrane conductance regulator chloride channel pore
Abstract
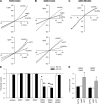
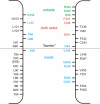
Different transmembrane (TM) α helices are known to line the pore of the cystic fibrosis TM conductance regulator (CFTR) Cl(-) channel. However, the relative alignment of these TMs in the three-dimensional structure of the pore is not known. We have used patch-clamp recording to investigate the accessibility of cytoplasmically applied cysteine-reactive reagents to cysteines introduced along the length of the pore-lining first TM (TM1) of a cysteine-less variant of CFTR. We find that methanethiosulfonate (MTS) reagents irreversibly modify cysteines substituted for TM1 residues K95, Q98, P99, and L102 when applied to the cytoplasmic side of open channels. Residues closer to the intracellular end of TM1 (Y84-T94) were not apparently modified by MTS reagents, suggesting that this part of TM1 does not line the pore. None of the internal MTS reagent-reactive cysteines was modified by extracellular [2-(trimethylammonium)ethyl] MTS. Only K95C, closest to the putative intracellular end of TM1, was apparently modified by intracellular [2-sulfonatoethyl] MTS before channel activation. Comparison of these results with recent work on CFTR-TM6 suggests a relative alignment of these two important TMs along the axis of the pore. This alignment was tested experimentally by formation of disulfide bridges between pairs of cysteines introduced into these two TMs. Currents carried by the double mutants K95C/I344C and Q98C/I344C, but not by the corresponding single-site mutants, were inhibited by the oxidizing agent copper(II)-o-phenanthroline. This inhibition was irreversible on washing but could be reversed by the reducing agent dithiothreitol, suggesting disulfide bond formation between the introduced cysteine side chains. These results allow us to develop a model of the relative positions, functional contributions, and alignment of two important TMs lining the CFTR pore. Such functional information is necessary to understand and interpret the three-dimensional structure of the pore.
Figures







References
-
- Akabas M.H., Kaufmann C., Cook T.A., Archdeacon P. 1994. Amino acid residues lining the chloride channel of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 269:14865–14868 - PubMed
-
- Alexander C., Ivetac A., Liu X., Norimatsu Y., Serrano J.R., Landstrom A., Sansom M., Dawson D.C. 2009. Cystic fibrosis transmembrane conductance regulator: using differential reactivity toward channel-permeant and channel-impermeant thiol-reactive probes to test a molecular model for the pore. Biochemistry. 48:10078–10088 10.1021/bi901314c - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

