Signal transduction by reactive oxygen species
- PMID: 21746850
- PMCID: PMC3135394
- DOI: 10.1083/jcb.201102095
Signal transduction by reactive oxygen species
Abstract
Although historically viewed as purely harmful, recent evidence suggests that reactive oxygen species (ROS) function as important physiological regulators of intracellular signaling pathways. The specific effects of ROS are modulated in large part through the covalent modification of specific cysteine residues found within redox-sensitive target proteins. Oxidation of these specific and reactive cysteine residues in turn can lead to the reversible modification of enzymatic activity. Emerging evidence suggests that ROS regulate diverse physiological parameters ranging from the response to growth factor stimulation to the generation of the inflammatory response, and that dysregulated ROS signaling may contribute to a host of human diseases.
Figures
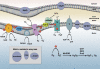
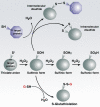
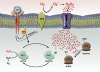
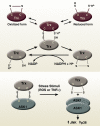
References
-
- Anderson E.J., Lustig M.E., Boyle K.E., Woodlief T.L., Kane D.A., Lin C.T., Price J.W., III, Kang L., Rabinovitch P.S., Szeto H.H., et al. 2009. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Invest. 119:573–581 10.1172/JCI37048 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

