α-Synuclein and ALPS motifs are membrane curvature sensors whose contrasting chemistry mediates selective vesicle binding
- PMID: 21746853
- PMCID: PMC3135411
- DOI: 10.1083/jcb.201011118
α-Synuclein and ALPS motifs are membrane curvature sensors whose contrasting chemistry mediates selective vesicle binding
Abstract
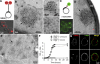
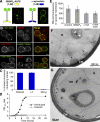
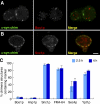
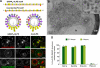
Membrane curvature sensors have diverse structures and chemistries, suggesting that they might have the intrinsic capacity to discriminate between different types of vesicles in cells. In this paper, we compare the in vitro and in vivo membrane-binding properties of two curvature sensors that form very different amphipathic helices: the amphipathic lipid-packing sensor (ALPS) motif of a Golgi vesicle tether and the synaptic vesicle protein α-synuclein, a causative agent of Parkinson's disease. We demonstrate the mechanism by which α-synuclein senses membrane curvature. Unlike ALPS motifs, α-synuclein has a poorly developed hydrophobic face, and this feature explains its dual sensitivity to negatively charged lipids and to membrane curvature. When expressed in yeast cells, these two curvature sensors were targeted to different classes of vesicles, those of the early secretory pathway for ALPS motifs and to negatively charged endocytic/post-Golgi vesicles in the case of α-synuclein. Through structures with complementary chemistries, α-synuclein and ALPS motifs target distinct vesicles in cells by direct interaction with different lipid environments.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

