Contribution of a Streptococcus mutans antigen expressed by a Salmonella vector vaccine in dendritic cell activation
- PMID: 21746857
- PMCID: PMC3165457
- DOI: 10.1128/IAI.05338-11
Contribution of a Streptococcus mutans antigen expressed by a Salmonella vector vaccine in dendritic cell activation
Abstract
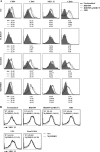
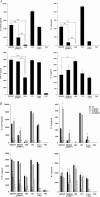
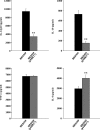
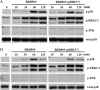
A Salmonella vector vaccine expressing the saliva-binding region (SBR) of the adhesin AgI/II of Streptococcus mutans has been shown to induce a mixed Th1/Th2 anti-SBR immune response in mice and to require Toll-like receptor 2 (TLR2), TLR4, and MyD88 signaling for the induction of mucosal anti-SBR antibody responses. Since dendritic cells (DC) are critical in innate and adaptive immunity, the present study assessed the role of SBR expression by the vector vaccine in DC activation. Bone marrow-derived DC from wild-type and TLR2, TLR4, and MyD88 knockout mice were stimulated with Salmonella vector BRD509, the SBR-expressing Salmonella vector vaccine BRD509(pSBRT7), or SBR protein, and the DC responses to different stimuli were compared by assessing costimulatory molecule expression, cytokine production, and signaling pathways. The DC response to both BRD509(pSBRT7) and BRD509 was dependent mainly on TLR4. BRD509(pSBRT7) and BRD509 induced upregulation of CD80, CD86, CD40, and major histocompatibility complex class II (MHC II) expression. Lower levels of interleukin-10 (IL-10) and IL-12p40 were produced by BRD509(pSBRT7)-stimulated DC than by BRD509-stimulated DC. Furthermore, BRD509(pSBRT7)-stimulated DC showed decreased p38 phosphorylation compared to that induced by DC stimulated with BRD509. However, BRD509(pSBRT7)-treated DC produced a higher level of IL-6 than BRD509-stimulated cells. The low IL-12p40 and high IL-6 cytokine profile expressed by BRD509(pSBRT7)-stimulated DC may represent a shift toward a Th2 response, as suggested by the increased expression in Jagged-1. These results provide novel evidence that a heterologous protein expressed by a Salmonella vector vaccine can differentially affect DC activation.
Figures

Similar articles
-
Role of Toll-like receptors in host responses to a virulence antigen of Streptococcus mutans expressed by a recombinant, attenuated Salmonella vector vaccine.Vaccine. 2010 Jul 12;28(31):4928-36. doi: 10.1016/j.vaccine.2010.05.039. Vaccine. 2010. PMID: 20653102 Free PMC article.
-
Requirement of TLR4 and CD14 in dendritic cell activation by Hemagglutinin B from Porphyromonas gingivalis.Mol Immunol. 2009 Aug;46(13):2493-504. doi: 10.1016/j.molimm.2009.05.022. Epub 2009 Jun 21. Mol Immunol. 2009. PMID: 19540594 Free PMC article.
-
Characterization of antigen-presenting cells induced by intragastric immunization with recombinant chimeric immunogens constructed from Streptococcus mutans AgI/II and type I or type II heat-labile enterotoxins.Mol Oral Microbiol. 2011 Jun;26(3):200-9. doi: 10.1111/j.2041-1014.2011.00608.x. Epub 2011 Mar 31. Mol Oral Microbiol. 2011. PMID: 21545697 Free PMC article.
-
Streptococcus mutans wall-associated protein A promotes TLR4-induced dendritic cell maturation.Scand J Immunol. 2014 Aug;80(2):121-6. doi: 10.1111/sji.12194. Scand J Immunol. 2014. PMID: 24846569
-
TLR4-mediated activation of dendritic cells by the heat shock protein DnaK from Francisella tularensis.J Leukoc Biol. 2008 Dec;84(6):1434-46. doi: 10.1189/jlb.0308215. Epub 2008 Aug 15. J Leukoc Biol. 2008. PMID: 18708593 Free PMC article.
Cited by
-
Identification of a supramolecular functional architecture of Streptococcus mutans adhesin P1 on the bacterial cell surface.J Biol Chem. 2015 Apr 3;290(14):9002-19. doi: 10.1074/jbc.M114.626663. Epub 2015 Feb 9. J Biol Chem. 2015. PMID: 25666624 Free PMC article.
-
Co-delivery of ccl19 gene enhances anti-caries DNA vaccine pCIA-P immunogenicity in mice by increasing dendritic cell migration to secondary lymphoid tissues.Acta Pharmacol Sin. 2013 Mar;34(3):432-40. doi: 10.1038/aps.2012.153. Epub 2013 Jan 21. Acta Pharmacol Sin. 2013. PMID: 23334235 Free PMC article.
-
Planktonic and Biofilm-Associated Pseudomonas aeruginosa and Staphylococcus epidermidis Elicit Differential Human Peripheral Blood Cell Responses.Microorganisms. 2021 Aug 31;9(9):1846. doi: 10.3390/microorganisms9091846. Microorganisms. 2021. PMID: 34576742 Free PMC article.
-
Binding forces of Streptococcus mutans P1 adhesin.ACS Nano. 2015 Feb 24;9(2):1448-60. doi: 10.1021/nn5058886. Epub 2015 Feb 11. ACS Nano. 2015. PMID: 25671413 Free PMC article.
-
Dormant bacteria within Staphylococcus epidermidis biofilms have low inflammatory properties and maintain tolerance to vancomycin and penicillin after entering planktonic growth.J Med Microbiol. 2014 Oct;63(Pt 10):1274-1283. doi: 10.1099/jmm.0.073163-0. Epub 2014 Jul 22. J Med Microbiol. 2014. PMID: 25053799 Free PMC article.
References
-
- Agnello D., et al. 2003. Cytokines and transcription factors that regulate T helper cell differentiation: new players and new insights. J. Clin. Immunol. 23:147–161 - PubMed
-
- Akira S., Takeda K. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499–511 - PubMed
-
- Amsen D., et al. 2004. Instruction of distinct CD4 T helper cell fates by different Notch ligands on antigen-presenting cells. Cell 117:515–526 - PubMed
-
- Banchereau J., et al. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767–811 - PubMed
-
- Banchereau J., Steinman R. M. 1998. Dendritic cells and the control of immunity. Nature 392:245–252 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

