Stanniocalcin 2 is a negative modulator of store-operated calcium entry
- PMID: 21746875
- PMCID: PMC3165734
- DOI: 10.1128/MCB.05140-11
Stanniocalcin 2 is a negative modulator of store-operated calcium entry
Abstract
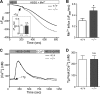
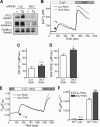
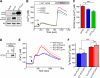
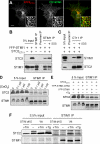
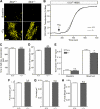
The regulation of cellular Ca(2+) homeostasis is essential for innumerable physiological and pathological processes. Stanniocalcin 1, a secreted glycoprotein hormone originally described in fish, is a well-established endocrine regulator of gill Ca(2+) uptake during hypercalcemia. While there are two mammalian Stanniocalcin homologs (STC1 and STC2), their precise molecular functions remain unknown. Notably, STC2 is a prosurvival component of the unfolded protein response. Here, we demonstrate a cell-intrinsic role for STC2 in the regulation of store-operated Ca(2+) entry (SOCE). Fibroblasts cultured from Stc2 knockout mice accumulate higher levels of cytosolic Ca(2+) following endoplasmic reticulum (ER) Ca(2+) store depletion, specifically due to an increase in extracellular Ca(2+) influx through store-operated Ca(2+) channels (SOC). The knockdown of STC2 expression in a hippocampal cell line also potentiates SOCE, and the overexpression of STC2 attenuates SOCE. Moreover, STC2 interacts with the ER Ca(2+) sensor STIM1, which activates SOCs following ER store depletion. These results define a novel molecular function for STC2 as a negative modulator of SOCE and provide the first direct evidence for the regulation of Ca(2+) homeostasis by mammalian STC2. Furthermore, our findings implicate the modulation of SOCE through STC2 expression as one of the prosurvival measures of the unfolded protein response.
Figures


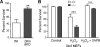
References
-
- Berridge M. J., Bootman M. D., Roderick H. L. 2003. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4:517–529 - PubMed
-
- Bertolotti A., Zhang Y., Hendershot L. M., Harding H. P., Ron D. 2000. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2:326–332 - PubMed
-
- Bolte S., Cordelieres F. P. 2006. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224:213–232 - PubMed
-
- Bouras T., et al. 2002. Stanniocalcin 2 is an estrogen-responsive gene coexpressed with the estrogen receptor in human breast cancer. Cancer Res. 62:1289–1295 - PubMed
-
- Buckanovich R. J., et al. 2007. Tumor vascular proteins as biomarkers in ovarian cancer. J. Clin. Oncol. 25:852–861 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
