A novel role of vimentin filaments: binding and stabilization of collagen mRNAs
- PMID: 21746880
- PMCID: PMC3165730
- DOI: 10.1128/MCB.05263-11
A novel role of vimentin filaments: binding and stabilization of collagen mRNAs
Abstract
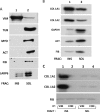
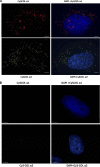
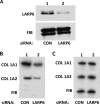
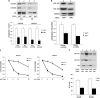
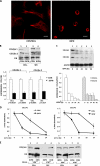
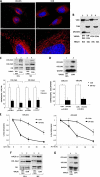
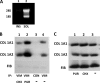
The stem-loop in the 5' untranslated region (UTR) of collagen α1(I) and α2(I) mRNAs (5'SL) is the key element regulating their stability and translation. Stabilization of collagen mRNAs is the predominant mechanism for high collagen expression in fibrosis. LARP6 binds the 5'SL of α1(I) and α2(I) mRNAs with high affinity. Here, we report that vimentin filaments associate with collagen mRNAs in a 5'SL- and LARP6-dependent manner and stabilize collagen mRNAs. LARP6 interacts with vimentin filaments through its La domain and colocalizes with the filaments in vivo. Knockdown of LARP6 by small interfering RNA (siRNA) or mutation of the 5'SL abrogates the interaction of collagen mRNAs with vimentin filaments. Vimentin knockout fibroblasts produce reduced amounts of type I collagen due to decreased stability of collagen α1(I) and α2(I) mRNAs. Disruption of vimentin filaments using a drug or by expression of dominant-negative desmin reduces type I collagen expression, primarily due to decreased stability of collagen mRNAs. RNA fluorescence in situ hybridization (FISH) experiments show that collagen α1(I) and α2(I) mRNAs are associated with vimentin filaments in vivo. Thus, vimentin filaments may play a role in the development of tissue fibrosis by stabilizing collagen mRNAs. This finding will serve as a rationale for targeting vimentin in the development of novel antifibrotic therapies.
Figures




References
-
- Anonymous. 2000. The American Association for the Study of Liver Diseases (AASLD) 51st annual meeting and postgraduate courses. October 27-31, 2000, Dallas, Texas, U. S. A. Abstracts. Hepatology 32:163A–655A - PubMed
-
- Bassell G., Singer R. H. 1997. mRNA and cytoskeletal filaments. Curr. Opin. Cell Biol. 9:109–115 - PubMed
-
- Bitterman P. B., Henke C. A. 1991. Fibroproliferative disorders. Chest 99:81S–84S - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
