1,25-dihydroxyvitamin D(3) ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A
- PMID: 21746882
- PMCID: PMC3165548
- DOI: 10.1128/MCB.05020-11
1,25-dihydroxyvitamin D(3) ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A
Abstract
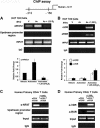
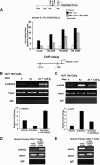
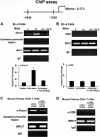
A new class of inflammatory CD4(+) T cells that produce interleukin-17 (IL-17) (termed Th17) has been identified, which plays a critical role in numerous inflammatory conditions and autoimmune diseases. The active form of vitamin D, 1,25-dihydroxyvitamin D(3) [1,25(OH)(2)D(3)], has a direct repressive effect on the expression of IL-17A in both human and mouse T cells. In vivo treatment of mice with ongoing experimental autoimmune encephalomyelitis (EAE; a mouse model of multiple sclerosis) diminishes paralysis and progression of the disease and reduces IL-17A-secreting CD4(+) T cells in the periphery and central nervous system (CNS). The mechanism of 1,25(OH)(2)D(3) repression of IL-17A expression was found to be transcriptional repression, mediated by the vitamin D receptor (VDR). Transcription assays, gel shifting, and chromatin immunoprecipitation (ChIP) assays indicate that the negative effect of 1,25(OH)(2)D(3) on IL-17A involves blocking of nuclear factor for activated T cells (NFAT), recruitment of histone deacetylase (HDAC), sequestration of Runt-related transcription factor 1 (Runx1) by 1,25(OH)(2)D(3)/VDR, and a direct effect of 1,25(OH)(2)D(3) on induction of Foxp3. Our results describe novel mechanisms and new concepts with regard to vitamin D and the immune system and suggest therapeutic targets for the control of autoimmune diseases.
Figures



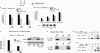

References
-
- Bhalla A. K., Amento E. P., Clemens T. L., Holick M. F., Krane S. M. 1983. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J. Clin. Endocrinol. Metab. 57:1308–1310 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
