Ligation of Fc gamma receptor IIB inhibits antibody-dependent enhancement of dengue virus infection
- PMID: 21746897
- PMCID: PMC3145677
- DOI: 10.1073/pnas.1106568108
Ligation of Fc gamma receptor IIB inhibits antibody-dependent enhancement of dengue virus infection
Abstract
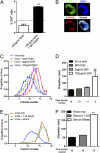
The interaction of antibodies, dengue virus (DENV), and monocytes can result in either immunity or enhanced virus infection. These opposing outcomes of dengue antibodies have hampered dengue vaccine development. Recent studies have shown that antibodies neutralize DENV by either preventing virus attachment to cellular receptors or inhibiting viral fusion intracellularly. However, whether the antibody blocks attachment or fusion, the resulting immune complexes are expected to be phagocytosed by Fc gamma receptor (FcγR)-bearing cells and cleared from circulation. This suggests that only antibodies that are able to block fusion intracellularly would be able to neutralize DENV upon FcγR-mediated uptake by monocytes whereas other antibodies would have resulted in enhancement of DENV replication. Using convalescent sera from dengue patients, we observed that neutralization of the homologous serotypes occurred despite FcγR-mediated uptake. However, FcγR-mediated uptake appeared to be inhibited when neutralized heterologous DENV serotypes were used instead. We demonstrate that this inhibition occurred through the formation of viral aggregates by antibodies in a concentration-dependent manner. Aggregation of viruses enabled antibodies to cross-link the inhibitory FcγRIIB, which is expressed at low levels but which inhibits FcγR-mediated phagocytosis and hence prevents antibody-dependent enhancement of DENV infection in monocytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Kyle JL, Harris E. Global spread and persistence of dengue. Annu Rev Microbiol. 2008;62:71–92. - PubMed
-
- Wilder-Smith A, Ooi EE, Vasudevan SG, Gubler DJ. Update on dengue: Epidemiology, virus evolution, antiviral drugs, and vaccine development. Curr Infect Dis Rep. 2010;12:157–164. - PubMed
-
- Halstead SB. Pathogenesis of dengue: Challenges to molecular biology. Science. 1988;239:476–481. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

