Structure of FcRY, an avian immunoglobulin receptor related to mammalian mannose receptors, and its complex with IgY
- PMID: 21746914
- PMCID: PMC3145690
- DOI: 10.1073/pnas.1106925108
Structure of FcRY, an avian immunoglobulin receptor related to mammalian mannose receptors, and its complex with IgY
Abstract
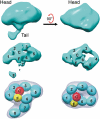
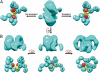
Fc receptors transport maternal antibodies across epithelial cell barriers to passively immunize newborns. FcRY, the functional counterpart of mammalian FcRn (a major histocompatibility complex homolog), transfers IgY across the avian yolk sac, and represents a new class of Fc receptor related to the mammalian mannose receptor family. FcRY and FcRn bind immunoglobulins at pH ≤6.5, but not pH ≥7, allowing receptor-ligand association inside intracellular vesicles and release at the pH of blood. We obtained structures of monomeric and dimeric FcRY and an FcRY-IgY complex and explored FcRY's pH-dependent binding mechanism using electron cryomicroscopy (cryoEM) and small-angle X-ray scattering. The cryoEM structure of FcRY at pH 6 revealed a compact double-ring "head," in which the N-terminal cysteine-rich and fibronectin II domains were folded back to contact C-type lectin-like domains 1-6, and a "tail" comprising C-type lectin-like domains 7-8. Conformational changes at pH 8 created a more elongated structure that cannot bind IgY. CryoEM reconstruction of FcRY dimers at pH 6 and small-angle X-ray scattering analysis at both pH values confirmed both structures. The cryoEM structure of the FcRY-IgY revealed symmetric binding of two FcRY heads to the dimeric FcY, each head contacting the C(H)4 domain of one FcY chain. FcRY shares structural properties with mannose receptor family members, including a head and tail domain organization, multimerization that may regulate ligand binding, and pH-dependent conformational changes. Our results facilitate understanding of immune recognition by the structurally related mannose receptor family and comparison of diverse methods of Ig transport across evolution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Roopenian DC, Akilesh S. FcRn: The neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–725. - PubMed
-
- Burmeister WP, Gastinel LN, Simister NE, Blum ML, Bjorkman PJ. Crystal structure at 2.2 A resolution of the MHC-related neonatal Fc receptor. Nature. 1994;372:336–343. - PubMed
-
- Simister NE, Mostov KE. An Fc receptor structurally related to MHC class I antigens. Nature. 1989;337:184–187. - PubMed
-
- Nimmerjahn F, Ravetch JV. Fcgamma receptors: Old friends and new family members. Immunity. 2006;24:19–28. - PubMed
-
- Woof JM, Burton DR. Human antibody-Fc receptor interactions illuminated by crystal structures. Nat Rev Immunol. 2004;4:89–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

