Plasticity of human auditory-evoked fields induced by shock conditioning and contingency reversal
- PMID: 21746922
- PMCID: PMC3145740
- DOI: 10.1073/pnas.1016124108
Plasticity of human auditory-evoked fields induced by shock conditioning and contingency reversal
Abstract
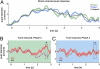
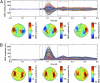
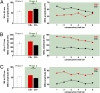
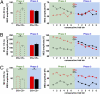
We used magnetoencephalography (MEG) to assess plasticity of human auditory cortex induced by classical conditioning and contingency reversal. Participants listened to random sequences of high or low tones. A first baseline phase presented these without further associations. In phase 2, one of the frequencies (CS(+)) was paired with shock on half its occurrences, whereas the other frequency (CS(-)) was not. In phase 3, the contingency assigning CS(+) and CS(-) was reversed. Conditioned pupil dilation was observed in phase 2 but extinguished in phase 3. MEG revealed that, during phase-2 initial conditioning, the P1m, N1m, and P2m auditory components, measured from sensors over auditory temporal cortex, came to distinguish between CS(+) and CS(-). After contingency reversal in phase 3, the later P2m component rapidly reversed its selectivity (unlike the pupil response) but the earlier P1m did not, whereas N1m showed some new learning but not reversal. These results confirm plasticity of human auditory responses due to classical conditioning, but go further in revealing distinct constraints on different levels of the auditory hierarchy. The later P2m component can reverse affiliation immediately in accord with an updated expectancy after contingency reversal, whereas the earlier auditory components cannot. These findings indicate distinct cognitive and emotional influences on auditory processing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Edeline JM, Weinberger NM. Receptive field plasticity in the auditory cortex during frequency discrimination training: Selective retuning independent of task difficulty. Behav Neurosci. 1993;107:82–103. - PubMed
-
- Bakin JS, Weinberger NM. Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Res. 1990;536:271–286. - PubMed
-
- Diamond DM, Weinberger NM. Classical conditioning rapidly induces specific changes in frequency receptive fields of single neurons in secondary and ventral ectosylvian auditory cortical fields. Brain Res. 1986;372:357–360. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

