Evasion of immunity to Plasmodium falciparum malaria by IgM masking of protective IgG epitopes in infected erythrocyte surface-exposed PfEMP1
- PMID: 21746929
- PMCID: PMC3145728
- DOI: 10.1073/pnas.1103708108
Evasion of immunity to Plasmodium falciparum malaria by IgM masking of protective IgG epitopes in infected erythrocyte surface-exposed PfEMP1
Abstract
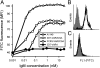
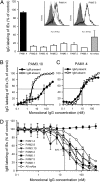
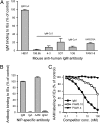
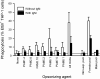
Plasmodium falciparum malaria is a major cause of mortality and severe morbidity. Its virulence is related to the parasite's ability to evade host immunity through clonal antigenic variation and tissue-specific adhesion of infected erythrocytes (IEs). The P. falciparum erythrocyte membrane protein 1 (PfEMP1) family is central to both. Here, we present evidence of a P. falciparum evasion mechanism not previously documented: the masking of PfEMP1-specific IgG epitopes by nonspecific IgM. Nonspecific IgM binding to erythrocytes infected by parasites expressing the PfEMP1 protein VAR2CSA (involved in placental malaria pathogenesis and protective immunity) blocked subsequent specific binding of human monoclonal IgG to the Duffy binding-like (DBL) domains DBL3X and DBL5ε of this PfEMP1 variant. Strikingly, a VAR2CSA-specific monoclonal antibody that binds outside these domains and can inhibit IE adhesion to the specific VAR2CSA receptor chondroitin sulfate A was unaffected. Nonspecific IgM binding protected the parasites from FcγR-dependent phagocytosis of VAR2CSA(+) IEs, but it did not affect IE adhesion to chondroitin sulfate A or lead to C1q deposition on IEs. Taken together, our results indicate that the VAR2CSA affinity for nonspecific IgM has evolved to allow placenta-sequestering P. falciparum to evade acquired protective immunity without compromising VAR2CSA function or increasing IE susceptibility to complement-mediated lysis. Furthermore, functionally important PfEMP1 epitopes not prone to IgM masking are likely to be particularly important targets of acquired protective immunity to P. falciparum malaria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Maitland K, Marsh K. Pathophysiology of severe malaria in children. Acta Trop. 2004;90:131–140. - PubMed
-
- Rogerson SJ, Hviid L, Duffy PE, Leke RF, Taylor DW. Malaria in pregnancy: Pathogenesis and immunity. Lancet Infect Dis. 2007;7:105–117. - PubMed
-
- Su XZ, et al. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82:89–100. - PubMed
-
- Hviid L. Naturally acquired immunity to Plasmodium falciparum malaria in Africa. Acta Trop. 2005;95:270–275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

