Related pituitary cell lineages develop into interdigitated 3D cell networks
- PMID: 21746936
- PMCID: PMC3145718
- DOI: 10.1073/pnas.1105929108
Related pituitary cell lineages develop into interdigitated 3D cell networks
Abstract
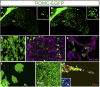
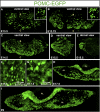
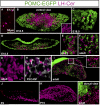
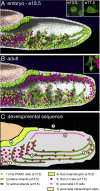
The pituitary gland has long been considered to be a random patchwork of hormone-producing cells. By using pituitary-scale tridimensional imaging for two of the least abundant cell lineages, the corticotropes and gonadotropes, we have now uncovered highly organized and interdigitated cell networks that reflect homotypic and heterotypic interactions between cells. Although newly differentiated corticotrope cells appear on the ventral surface of the gland, they rapidly form homotypic strands of cells that extend from the lateral tips of the anterior pituitary along its ventral surface and into the medial gland. As the corticotrope network is established away from the microvasculature, cell morphology changes from rounded, to polygonal, and finally to cells with long cytoplasmic processes or cytonemes that connect corticotropes to the perivascular space. Gonadotropes differentiate later and are positioned in close proximity to corticotropes and capillaries. Blockade of corticotrope terminal differentiation produced by knockout of the gene encoding the transcription factor Tpit results in smaller gonadotropes within an expanded cell network, particularly in the lateral gland. Thus, pituitary-scale tridimensional imaging reveals highly structured cell networks of unique topology for each pituitary lineage. The sequential development of interdigitated cell networks during organogenesis indicate that extensive cell:cell interactions lead to a highly ordered cell positioning rather than random patchwork.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Melmed S, Drouin J. Pituitary development. In: Melmed S, editor. The Pituitary. Oxford: Academic Press; 2010. pp. 3–19.
-
- Harris GW. The function of the pituitary stalk. Bull Johns Hopkins Hosp. 1955;97:358–375. - PubMed
-
- Schally AV. Aspects of hypothalamic regulation of the pituitary gland. Science. 1978;202:18–28. - PubMed
-
- Guillemin R. Peptides in the brain: The new endocrinology of the neuron. Science. 1978;202:390–402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

