The Src family kinase Fgr is critical for activation of mast cells and IgE-mediated anaphylaxis in mice
- PMID: 21746961
- PMCID: PMC3163437
- DOI: 10.4049/jimmunol.1100296
The Src family kinase Fgr is critical for activation of mast cells and IgE-mediated anaphylaxis in mice
Abstract
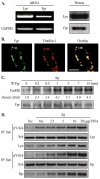
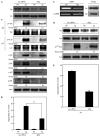
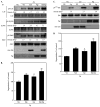
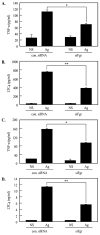
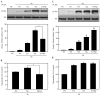
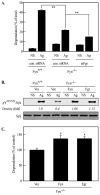
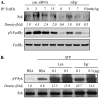
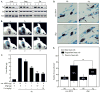
Mast cells are critical for various allergic disorders. Mast cells express Src family kinases, which relay positive and negative regulatory signals by Ag. Lyn, for example, initiates activating signaling events, but it also induces inhibitory signals. Fyn and Hck are reported to be positive regulators, but little is known about the roles of other Src kinases, including Fgr, in mast cells. In this study, we define the role of Fgr. Endogenous Fgr associates with FcεRI and promotes phosphorylation of Syk, Syk substrates, which include linkers for activation of T cells, SLP76, and Gab2, and downstream targets such as Akt and the MAPKs in Ag-stimulated mast cells. As a consequence, Fgr positively regulates degranulation, production of eicosanoids, and cytokines. Fgr and Fyn appeared to act in concert, as phosphorylation of Syk and degranulation are enhanced by overexpression of Fgr and further augmented by overexpression of Fyn but are suppressed by overexpression of Lyn. Moreover, knockdown of Fgr by small interfering RNAs (siRNAs) further suppressed degranulation in Fyn-deficient bone marrow-derived mast cells. Overexpression of Fyn or Fgr restored phosphorylation of Syk and partially restored degranulation in Fyn-deficient cells. Additionally, knockdown of Fgr by siRNAs inhibited association of Syk with FcεRIγ as well as the tyrosine phosphorylation of FcεRIγ. Of note, the injection of Fgr siRNAs diminished the protein level of Fgr in mice and simultaneously inhibited IgE-mediated anaphylaxis. In conclusion, Fgr positively regulates mast cell through activation of Syk. These findings help clarify the interplay among Src family kinases and identify Fgr as a potential therapeutic target for allergic diseases.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures
References
-
- Bischoff SC. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nat Rev Immunol. 2007;7:93–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

