Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus
- PMID: 21747057
- PMCID: PMC3149100
- DOI: 10.1161/CIRCULATIONAHA.110.014506
Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus
Abstract
Background: Endothelial dysfunction contributes to the development of atherosclerosis in patients with diabetes mellitus, but the mechanisms of endothelial dysfunction in this setting are incompletely understood. Recent studies have shown altered mitochondrial dynamics in diabetes mellitus with increased mitochondrial fission and production of reactive oxygen species. We investigated the contribution of altered dynamics to endothelial dysfunction in diabetes mellitus.
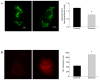
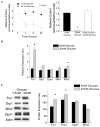
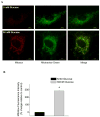
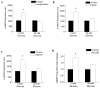
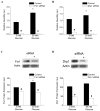
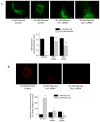
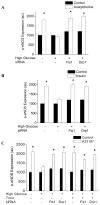
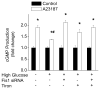
Methods and results: We observed mitochondrial fragmentation (P=0.002) and increased expression of fission-1 protein (Fis1; P<0.0001) in venous endothelial cells freshly isolated from patients with diabetes mellitus (n=10) compared with healthy control subjects (n=9). In cultured human aortic endothelial cells exposed to 30 mmol/L glucose, we observed a similar loss of mitochondrial networks and increased expression of Fis1 and dynamin-related protein-1 (Drp1), proteins required for mitochondrial fission. Altered mitochondrial dynamics was associated with increased mitochondrial reactive oxygen species production and a marked impairment of agonist-stimulated activation of endothelial nitric oxide synthase and cGMP production. Silencing Fis1 or Drp1 expression with siRNA blunted high glucose-induced alterations in mitochondrial networks, reactive oxygen species production, endothelial nitric oxide synthase activation, and cGMP production. An intracellular reactive oxygen species scavenger provided no additional benefit, suggesting that increased mitochondrial fission may impair endothelial function via increased reactive oxygen species.
Conclusion: These findings implicate increased mitochondrial fission as a contributing mechanism for endothelial dysfunction in diabetic states.
Figures
References
-
- Center for Disease Control and Prevention. 2007 National Diabetes Fact Sheet. 2007.
-
- Tesfamariam B, Cohen RA. Free radicals mediate endothelial cell dysfunction caused by elevated glucose. Am J Physiol. 1992;263:H321–H326. - PubMed
-
- Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–1945. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K23HL089326/HL/NHLBI NIH HHS/United States
- K23 HL089326/HL/NHLBI NIH HHS/United States
- HL081587/HL/NHLBI NIH HHS/United States
- P50 HL083801/HL/NHLBI NIH HHS/United States
- R01 HL083269/HL/NHLBI NIH HHS/United States
- R01 DK074778/DK/NIDDK NIH HHS/United States
- K12 HL083781/HL/NHLBI NIH HHS/United States
- DK035914/DK/NIDDK NIH HHS/United States
- HL083801/HL/NHLBI NIH HHS/United States
- P01 HL081587/HL/NHLBI NIH HHS/United States
- R56 DK035914/DK/NIDDK NIH HHS/United States
- P01 HL060886/HL/NHLBI NIH HHS/United States
- DK074778/DK/NIDDK NIH HHS/United States
- R01 HL075795/HL/NHLBI NIH HHS/United States
- R01 DK035914/DK/NIDDK NIH HHS/United States
- HL102299/HL/NHLBI NIH HHS/United States
- HL75795/HL/NHLBI NIH HHS/United States
- HL083269/HL/NHLBI NIH HHS/United States
- R01 HL102299/HL/NHLBI NIH HHS/United States
- R01 DK056690/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

