Impact of treatment end point definitions on perceived differences in long-term outcome with tyrosine kinase inhibitor therapy in chronic myeloid leukemia
- PMID: 21747082
- PMCID: PMC4874216
- DOI: 10.1200/JCO.2010.33.4169
Impact of treatment end point definitions on perceived differences in long-term outcome with tyrosine kinase inhibitor therapy in chronic myeloid leukemia
Abstract
Purpose: Different definitions of progression-free survival (PFS) and event-free survival (EFS) may result in perceived differences in outcomes with tyrosine kinase inhibitor (TKI) therapies in chronic myelogenous leukemia (CML).
Patients and methods: We analyzed the outcome of 435 patients with early chronic-phase, Philadelphia chromosome-positive CML treated with imatinib (n = 281), nilotinib (n = 78), and dasatinib (n = 76) using definitions of PFS and EFS used in the International Randomized Study of Interferon Versus STI571 (IRIS), Evaluating Nilotinib Efficacy and Safety in Clinical Trials-Newly Diagnosed Patients (ENEST-nd), Dasatinib Versus Imatinib Study in Treatment-Naïve CML Patients (DASISION), and MD Anderson Cancer Center (MDACC) trials. Definitions for EFS-IRIS, time without progression in ENEST-nd, PFS-DASISION, and EFS-MDACC were as previously reported. The EFS-MDACC considered an event any instance of toxicity or death from any cause on or off therapy (if not counted before death as progression/event).
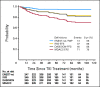
Results: Of the 435 patients, 123 (28%) were taken off TKI therapy (resistance/loss of response, n = 33; blastic phase on TKI therapy, n = 6; intolerance/toxicity, n = 29; other causes, n = 55). Thirty-three patients (7.6%) have died; eight patients died on TKI therapy, two patients died within 60 days of being off TKIs, and 23 patients died after being off TKIs for more than 60 days. Of the 33 deaths, 19 deaths (eight deaths on TKI, two deaths within 60 days, and nine deaths off for resistance/relapse/transformation) would be counted as progression/events on the IRIS/ENEST-nd/DASISION studies, whereas 14 deaths would be censored at time off TKI. On the basis of the four definitions used by IRIS, ENEST-nd, DASISION, and MDACC trials, the corresponding 5-year PFS/EFS rates were 96%, 90%, 89%, and 81%.
Conclusion: Uniform definitions of PFS and EFS are needed to compare the long-term efficacy and potential use of different TKIs in CML.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


References
-
- O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. - PubMed
-
- Kantarjian H, Talpaz M, O'Brien S, et al. Survival benefit with imatinib mesylate versus interferon-alpha-based regimens in newly diagnosed chronic-phase chronic myelogenous leukemia. Blood. 2006;108:1835–1840. - PubMed
-
- Cortes J, Silver R, Kantarjian H. Chronic myeloid leukemia. In: Hong W, Bast R, Hait W, et al., editors. Cancer Medicine. ed 8. Shelton, CT: People's Medical Publishing House; 2010. pp. 1582–1590.
-
- Kantarjian H, O'Brien S, Cortes J, et al. Complete cytogenetic and molecular responses to interferon-alpha-based therapy for chronic myelogenous leukemia are associated with excellent long-term prognosis. Cancer. 2003;97:1033–1041. - PubMed
-
- Hughes TP, Kaeda J, Branford S, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349:1423–1432. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

