Documenting the natural history of patients with resected stage II adenocarcinoma of the colon after random assignment to adjuvant treatment with edrecolomab or observation: results from CALGB 9581
- PMID: 21747085
- PMCID: PMC3157980
- DOI: 10.1200/JCO.2010.32.5357
Documenting the natural history of patients with resected stage II adenocarcinoma of the colon after random assignment to adjuvant treatment with edrecolomab or observation: results from CALGB 9581
Abstract
Purpose: We conducted a randomized trial comparing adjuvant treatment with edrecolomab versus observation in patients with resected, low-risk, stage II colon cancer. This study also prospectively studied patient- and tumor-specific markers of treatment outcome.
Patients and methods: After surgical resection, patients with stage II colon cancer were randomly assigned to either five infusions of edrecolomab at 28-day intervals or observation without adjuvant therapy.
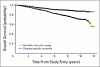
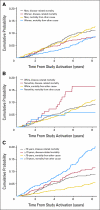
Results: Final accrual included 1,738 patients; 865 patients received edrecolomab, and 873 patients were observed without adjuvant treatment. Median follow-up time was 7.9 years. There were no significant outcome differences between study arms (overall survival [OS], P = .71; disease-free survival, P = .64). The combined 5-year all-cause OS was 0.86 (95% CI, 0.84 to 0.88), and the combined 5-year disease-specific OS was 0.93 (95% CI, 0.91 to 0.94). The relationships between demographic and histopathologic factors and survival differed for all-cause and disease-specific survival outcomes, but no combined prognostic factor model was found to adequately classify patients at higher risk of recurrence or death as a result of colon cancer.
Conclusion: Edrecolomab did not prolong survival. Consequently, this large study with a long duration of follow-up provided unique data concerning the natural history of resected stage II colon cancer. Prognostic factors identified in previous retrospective and pooled analyses were associated with survival outcomes in this stage II patient cohort. Results from ongoing molecular marker studies may enhance our ability to determine the risk profile of these patients.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. - PubMed
-
- Wolmark N, Rockette H, Fisher B, et al. The benefit of leucovorin-modulated fluorouracil as postoperative adjuvant therapy for primary colon cancer: Results from National Surgical Adjuvant Breast and Bowel Project protocol C-03. J Clin Oncol. 1993;11:1879–1887. - PubMed
-
- Efficacy of adjuvant fluorouracil and folinic acid in colon cancer: International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) Investigators. Lancet. 1995;345:939–944. - PubMed
-
- Haller DG, Catalano PJ, Macdonald JS, et al. Fluorouracil (FU), leucovorin (LV) and levamisole (LEV) adjuvant therapy for colon cancer: Five-year final report of INT-0089. Proc Am Soc Clin Oncol. 1998;17(suppl):256a. abstr 982.
-
- Zaniboni A. Adjuvant chemotherapy in colorectal cancer with high-dose leucovorin and fluorouracil: Impact on disease-free survival and overall survival. J Clin Oncol. 1997;15:2432–2441. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

