Unique sphingomyelin patches are targets of a beta-cell-specific antibody
- PMID: 21747097
- PMCID: PMC3151686
- DOI: 10.1194/jlr.M017582
Unique sphingomyelin patches are targets of a beta-cell-specific antibody
Abstract
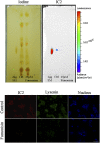
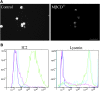
To devise successful imaging and therapeutic strategies, the identification of β-cell surface markers is one of the challenges in diabetes research that has to be resolved. We previously showed that IC2, a rat monoclonal IgM antibody, can be used for ex vivo determination of β-cell mass by imaging. Further progress toward the development of an antibody-based imaging agent was hampered by the lack of knowledge regarding the nature and composition of the IC2 antigen. Here, we show a series of systematic experiments involving classical lipid extraction and chromatography techniques combined with immunochemistry, which led to the identification of sphingomyelin as the target antigen assembled in the form of patches on the β-cell surface. Our findings were verified by modulating SM by enzymatic cleavage, downregulation, upregulation, and perturbation of membrane SM and observation of corresponding changes in IC2 binding. Cholesterol participates in stabilization of these patches, as its removal results in loss of IC2 binding. We believe that these findings have implications for identifying future ligands for the proposed antigen for imaging purposes as well as for potential therapy, as sphingomyelin has been shown to play a role in the apoptotic cascade in pancreatic β cells.
Figures
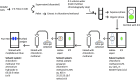

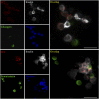
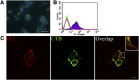



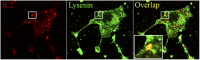

References
-
- Moore A., Bonner-Weir S., Weissleder R. 2001. Noninvasive in vivo measurement of beta-cell mass in mouse model of diabetes. Diabetes. 50: 2231–2236. - PubMed
-
- Kung M., Hou C., Lieberman B., Oya S., Ponde D., Blankemeyer E., Skovronsky D., Kilbourn M. R., Kung H. 2008. In vivo imaging of β-cell mass in rats using 18F-FP-(+)-DTBZ: a potential PET ligand for studying diabetes mellitus. J. Nucl. Med. 49: 1171–1176. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

