Pregnane X receptor activation induces FGF19-dependent tumor aggressiveness in humans and mice
- PMID: 21747170
- PMCID: PMC3148718
- DOI: 10.1172/JCI41514
Pregnane X receptor activation induces FGF19-dependent tumor aggressiveness in humans and mice
Abstract
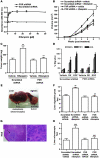
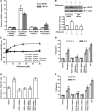
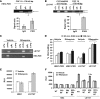
The nuclear receptor pregnane X receptor (PXR) is activated by a range of xenochemicals, including chemotherapeutic drugs, and has been suggested to play a role in the development of tumor cell resistance to anticancer drugs. PXR also has been implicated as a regulator of the growth and apoptosis of colon tumors. Here, we have used a xenograft model of colon cancer to define a molecular mechanism that might underlie PXR-driven colon tumor growth and malignancy. Activation of PXR was found to be sufficient to enhance the neoplastic characteristics, including cell growth, invasion, and metastasis, of both human colon tumor cell lines and primary human colon cancer tissue xenografted into immunodeficient mice. Furthermore, we were able to show that this PXR-mediated phenotype required FGF19 signaling. PXR bound to the FGF19 promoter in both human colon tumor cells and "normal" intestinal crypt cells. However, while both cell types proliferated in response to PXR ligands, the FGF19 promoter was activated by PXR only in cancer cells. Taken together, these data indicate that colon cancer growth in the presence of a specific PXR ligand results from tumor-specific induction of FGF19. These observations may lead to improved therapeutic regimens for colon carcinomas.
Figures




Similar articles
-
Lithocholic acid induction of the FGF19 promoter in intestinal cells is mediated by PXR.World J Gastroenterol. 2007 Aug 21;13(31):4230-5. doi: 10.3748/wjg.v13.i31.4230. World J Gastroenterol. 2007. PMID: 17696253 Free PMC article.
-
Pregnane X receptor suppresses proliferation and tumourigenicity of colon cancer cells.Br J Cancer. 2010 Jun 8;102(12):1753-61. doi: 10.1038/sj.bjc.6605677. Br J Cancer. 2010. PMID: 20531417 Free PMC article.
-
Activation of intestinal human pregnane X receptor protects against azoxymethane/dextran sulfate sodium-induced colon cancer.J Pharmacol Exp Ther. 2014 Dec;351(3):559-67. doi: 10.1124/jpet.114.215913. Epub 2014 Oct 2. J Pharmacol Exp Ther. 2014. PMID: 25277138 Free PMC article.
-
Pregnane xenobiotic receptor in cancer pathogenesis and therapeutic response.Cancer Lett. 2013 Jan 1;328(1):1-9. doi: 10.1016/j.canlet.2012.08.030. Epub 2012 Aug 29. Cancer Lett. 2013. PMID: 22939994 Free PMC article. Review.
-
Role of pregnane X receptor in chemotherapeutic treatment.Cancer Chemother Pharmacol. 2014 Aug;74(2):217-27. doi: 10.1007/s00280-014-2494-9. Epub 2014 Jun 3. Cancer Chemother Pharmacol. 2014. PMID: 24889719 Free PMC article. Review.
Cited by
-
Allosteric Antagonism of the Pregnane X Receptor (PXR): Current-State-of-the-Art and Prediction of Novel Allosteric Sites.Cells. 2022 Sep 24;11(19):2974. doi: 10.3390/cells11192974. Cells. 2022. PMID: 36230936 Free PMC article. Review.
-
Selective Agonism of Liver and Gut FXR Prevents Cholestasis and Intestinal Atrophy in Parenterally Fed Neonatal Pigs.bioRxiv [Preprint]. 2024 Sep 7:2024.09.03.611073. doi: 10.1101/2024.09.03.611073. bioRxiv. 2024. PMID: 39282416 Free PMC article. Preprint.
-
PXR variants: the impact on drug metabolism and therapeutic responses.Acta Pharm Sin B. 2016 Sep;6(5):441-449. doi: 10.1016/j.apsb.2016.07.002. Epub 2016 Jul 27. Acta Pharm Sin B. 2016. PMID: 27709012 Free PMC article. Review.
-
Intestinal pregnane X receptor links xenobiotic exposure and hypercholesterolemia.Mol Endocrinol. 2015 May;29(5):765-76. doi: 10.1210/me.2014-1355. Epub 2015 Mar 26. Mol Endocrinol. 2015. PMID: 25811240 Free PMC article.
-
Expression of NR1I3 in mouse lung tumors induced by the tobacco-specific nitrosamine 4-(methylnitrosamino)-4-(3-pyridyl)-1-butanone.Braz J Med Biol Res. 2015 Mar;48(3):240-4. doi: 10.1590/1414-431X20144210. Epub 2015 Feb 13. Braz J Med Biol Res. 2015. PMID: 25714878 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

