Angiotensin II, oxidative stress and skeletal muscle wasting
- PMID: 21747283
- PMCID: PMC3217236
- DOI: 10.1097/MAJ.0b013e318222e620
Angiotensin II, oxidative stress and skeletal muscle wasting
Abstract
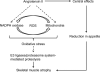
Muscle atrophy (cachexia) is a muscle wasting syndrome associated with several pathological conditions in humans such as congestive heart failure, diabetes, AIDS, cancer and renal failure, and the presence of cachexia worsens outcome. Many of the conditions associated with cachexia are accompanied by stimulation of the renin-angiotensin system and elevation in angiotensin II (ang II) levels. Ang II infusion induces skeletal muscle atrophy in rodents and mechanisms include increased expression of the E3 ligases atrogin-1/MuRF-1, an elevated rate of ubiquitin-proteasome mediated proteolysis and increased reactive oxygen species (ROS) levels, closely mimicking conditions of human cachexia. Ang II-induced oxidative stress contributes to muscle atrophy in a mouse model. Nicotinamide adenine dinucleotide phosphate oxidase- and mitochondria-derived ROS contribute to ang II-induced oxidative stress. Specific targeting of ROS and nicotinamide adenine dinucleotide phosphate oxidase/mitochondria cross-talk could be a beneficial, novel therapy to treat cachexia.
Figures
References
-
- Anker SD, Ponikowski P, Varney S, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–3. - PubMed
-
- Marcell TJ. Sarcopenia: causes, consequences, and preventions. J Gerontol A Biol Sci Med Sci. 2003;58:M911–6. - PubMed
-
- Glass DJ. Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol. 2003;5:87–90. - PubMed
-
- Bodine SC, Latres E, Baumhueter S, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

