Chlamydia trachomatis immune evasion via downregulation of MHC class I surface expression involves direct and indirect mechanisms
- PMID: 21747639
- PMCID: PMC3123996
- DOI: 10.1155/2011/420905
Chlamydia trachomatis immune evasion via downregulation of MHC class I surface expression involves direct and indirect mechanisms
Abstract
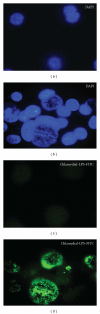
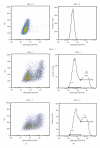
Genital C. trachomatis infections typically last for many months in women. This has been attributed to several strategies by which C. trachomatis evades immune detection, including well-described methods by which C. trachomatis decreases the cell surface expression of the antigen presenting molecules major histocompatibility complex (MHC) class I, MHC class II, and CD1d in infected genital epithelial cells. We have harnessed new methods that allow for separate evaluation of infected and uninfected cells within a mixed population of chlamydia-infected endocervical epithelial cells to demonstrate that MHC class I downregulation in the presence of C. trachomatis is mediated by direct and indirect (soluble) factors. Such indirect mechanisms may aid in priming surrounding cells for more rapid immune evasion upon pathogen entry and help promote unfettered spread of C. trachomatis genital infections.
Figures

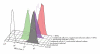
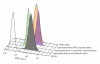
References
-
- AbdelRahman YM, Belland RJ. The chlamydial developmental cycle. FEMS Microbiology Reviews. 2005;29(5):949–959. - PubMed
-
- Wyrick PB, Davis CH, Knight ST, Choong J, Raulston JE, Schramm N. An in vitro human epithelial cell culture system for studying the pathogenesis of Chlamydia trachomatis. Sexually Transmitted Diseases. 1993;20(5):248–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

