Proliferation-attenuating and apoptosis-inducing effects of tryptanthrin on human chronic myeloid leukemia K562 cell line in vitro
- PMID: 21747710
- PMCID: PMC3131594
- DOI: 10.3390/ijms12063831
Proliferation-attenuating and apoptosis-inducing effects of tryptanthrin on human chronic myeloid leukemia K562 cell line in vitro
Abstract
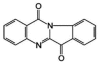
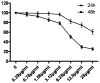
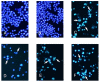
Tryptanthrin, a kind of indole quinazoline alkaloid, has been shown to exhibit anti-microbial, anti-inflammation and anti-tumor effects both in vivo and in vitro. However, its biological activity on human chronic myeloid leukemia cell line K562 is not fully understood. In the present study, we investigated the proliferation-attenuating and apoptosis-inducing effects of tryptanthrin on leukemia K562 cells in vitro and explored the underlying mechanisms. The results showed that tryptanthrin could significantly inhibit K562 cells proliferation in a time- and dose-dependent manner as evidenced by MTT assay and flow cytometry analysis. We also observed pyknosis, chromatin margination and the formation of apoptotic bodies in the presence of tryptanthrin under the electron microscope. Nuclei fragmentation and condensation by Hoechst 33258 staining were detected as well. The amount of apoptotic cells significantly increased whereas the mitochondrial membrane potential decreased dramatically after tryptanthrin exposure. K562 cells in the tryptanthrin treated group exhibited an increase in cytosol cyt-c, Bax and activated caspase-3 expression while a decrease in Bcl-2, mito cyt-c and pro-caspase-3 contents. However, the changes of pro-caspase-3 and activated caspase-3 could be abolished by a pan-caspase inhibitor ZVAD-FMK. These results suggest that tryptanthrin has proliferation-attenuating and apoptosis-inducing effects on K562 cells. The underlying mechanism is probably attributed to the reduction in mitochondria membrane potential, the release of mito cyt-c and pro-caspase-3 activation.
Keywords: K562 cells; apoptosis; chronic myeloid leukemia; proliferation; tryptanthrin.
Figures


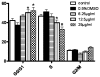



References
-
- Shim MJ, Kim HJ, Yang SJ, Lee IS, Choi HI, Kim T. Arsenic trioxide induces apoptosis in chronic myelogenous leukemia K562 cells: Possible involvement of p38 MAP kinase. J. Biochem. Mol. Biol. 2002;35:377–383. - PubMed
-
- Zhang Y, Dawson MI, Mohammad R, Rishi AK, Farhana L, Feng KC, Leid M, Peterson V, Zhang XK, Edelstein M, et al. Induction of apoptosis of human B-CLL and ALL cells by a novel retinoid and its nonretinoidal analog. Blood. 2002;100:2917–2925. - PubMed
-
- Darzynkiewicz Z, Juan G, Li X, Gorczyca W, Murakami T, Traganos F. Cytometry in cell necrobiology: Analysis of apoptosis and accidental cell death (necrosis) Cytometry. 1997;27:1–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

