Post-Transcriptional Control of the Hypoxic Response by RNA-Binding Proteins and MicroRNAs
- PMID: 21747757
- PMCID: PMC3130151
- DOI: 10.3389/fnmol.2011.00007
Post-Transcriptional Control of the Hypoxic Response by RNA-Binding Proteins and MicroRNAs
Abstract
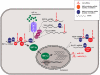
Mammalian gene expression patterns change profoundly in response to low oxygen levels. These changes in gene expression programs are strongly influenced by post-transcriptional mechanisms mediated by mRNA-binding factors: RNA-binding proteins (RBPs) and microRNAs (miRNAs). Here, we review the RBPs and miRNAs that modulate mRNA turnover and translation in response to hypoxic challenge. RBPs such as HuR (human antigen R), PTB (polypyrimidine tract-binding protein), heterogeneous nuclear ribonucleoproteins (hnRNPs), tristetraprolin, nucleolin, iron-response element-binding proteins (IRPs), and cytoplasmic polyadenylation-element-binding proteins (CPEBs), selectively bind to numerous hypoxia-regulated transcripts and play a major role in establishing hypoxic gene expression patterns. MiRNAs including miR-210, miR-373, and miR-21 associate with hypoxia-regulated transcripts and further modulate the levels of the encoded proteins to implement the hypoxic gene expression profile. We discuss the potent regulation of hypoxic gene expression by RBPs and miRNAs and their integrated actions in the cellular hypoxic response.
Keywords: RNA-binding proteins; hypoxia; mRNA turnover; microRNAs; post-transcriptional gene regulation; ribonucleoprotein complex; translational control; untranslated regions.
Figures
Similar articles
-
RNA-binding proteins implicated in the hypoxic response.J Cell Mol Med. 2009 Sep;13(9A):2759-69. doi: 10.1111/j.1582-4934.2009.00842.x. Epub 2009 Jul 6. J Cell Mol Med. 2009. PMID: 19583805 Free PMC article. Review.
-
Regulation of nucleolin expression by miR-194, miR-206, and HuR.Mol Cell Biochem. 2016 Jun;417(1-2):141-53. doi: 10.1007/s11010-016-2721-2. Epub 2016 May 25. Mol Cell Biochem. 2016. PMID: 27221739
-
Post-transcriptional regulation of programmed cell death 4 (PDCD4) mRNA by the RNA-binding proteins human antigen R (HuR) and T-cell intracellular antigen 1 (TIA1).J Biol Chem. 2015 Feb 6;290(6):3468-87. doi: 10.1074/jbc.M114.631937. Epub 2014 Dec 17. J Biol Chem. 2015. PMID: 25519906 Free PMC article.
-
HuR and other turnover- and translation-regulatory RNA-binding proteins: implications for the kidney.Am J Physiol Renal Physiol. 2014 Mar 15;306(6):F569-76. doi: 10.1152/ajprenal.00270.2013. Epub 2014 Jan 15. Am J Physiol Renal Physiol. 2014. PMID: 24431206 Review.
-
Posttranscriptional Regulation of Intestinal Epithelial Tight Junction Barrier by RNA-binding Proteins and microRNAs.Tissue Barriers. 2014 Jan 1;2(1):e28320. doi: 10.4161/tisb.28320. Epub 2014 Mar 19. Tissue Barriers. 2014. PMID: 24843843 Free PMC article. Review.
Cited by
-
Exploitation of tolerance to drought stress in carrot (Daucus carota L.): an overview.Stress Biol. 2023 Dec 11;3(1):55. doi: 10.1007/s44154-023-00130-0. Stress Biol. 2023. PMID: 38079026 Free PMC article. Review.
-
New insights into functional roles of the polypyrimidine tract-binding protein.Int J Mol Sci. 2013 Nov 20;14(11):22906-32. doi: 10.3390/ijms141122906. Int J Mol Sci. 2013. PMID: 24264039 Free PMC article. Review.
-
Holding our breath: The emerging and anticipated roles of microRNA in pulmonary hypertension.Pulm Circ. 2012 Jul;2(3):278-90. doi: 10.4103/2045-8932.101395. Pulm Circ. 2012. PMID: 23130098 Free PMC article.
-
Hypoxia negatively regulates antimetastatic PEDF in melanoma cells by a hypoxia inducible factor-independent, autophagy dependent mechanism.PLoS One. 2012;7(3):e32989. doi: 10.1371/journal.pone.0032989. Epub 2012 Mar 23. PLoS One. 2012. PMID: 22457728 Free PMC article.
-
Multilevel regulation of HIF-1 signaling by TTP.Mol Biol Cell. 2012 Oct;23(20):4129-41. doi: 10.1091/mbc.E11-11-0949. Epub 2012 Aug 23. Mol Biol Cell. 2012. PMID: 22918951 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

