Intra-Vacuolar Proliferation of F. Novicida within H. Vermiformis
- PMID: 21747796
- PMCID: PMC3128938
- DOI: 10.3389/fmicb.2011.00078
Intra-Vacuolar Proliferation of F. Novicida within H. Vermiformis
Abstract
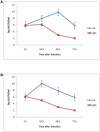
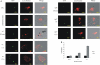
Francisella tularensis is a gram negative facultative intracellular bacterium that causes the zoonotic disease tularemia. Free-living amebae, such as Acanthamoeba and Hartmannella, are environmental hosts of several intracellular pathogens. Epidemiology of F. tularensis in various parts of the world is associated with water-borne transmission, which includes mosquitoes and amebae as the potential host reservoirs of the bacteria in water resources. In vitro studies showed intracellular replication of F. tularensis within A. castellanii cells. Whether ameba is a biological reservoir for Francisella in the environment is not known. We used Hartmannella vermiformis as an amebal model system to study the intracellular life of F. novicida. For the first time we show that F. novicida survives and replicates within H. vermiformis. The iglC mutant strain of F. novicida is defective for survival and replication not only within A. castellanii but also in H. vermiformis cells. In contrast to mammalian cells, where bacteria replicate in the cytosol, F. novicida resides and replicates within membrane-bound vacuoles within the trophozoites of H. vermiformis. In contrast to the transient residence of F. novicida within acidic vacuoles prior to escaping to the cytosol of mammalian cells, F. novicida does not reside transiently or permanently in an acidic compartment within H. vermiformis when examined 30 min after initiation of the infection. We conclude that F. tularensis does not replicate within acidified vacuoles and does not escape into the cytosol of H. vermiformis. The Francisella pathogenicity island locus iglC is essential for intra-vacuolar proliferation of F. novicida within H. vermiformis. Our data show a distinct intracellular lifestyle for F. novicida within H. vermiformis compared to mammalian cells.
Keywords: Francisella novicida; Hartmannella vermiformis; LysoTracker; iglC; vacuolar replication.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

