Estradiol-Treated Female Mice as Surrogate Hosts for Neisseria gonorrhoeae Genital Tract Infections
- PMID: 21747807
- PMCID: PMC3129519
- DOI: 10.3389/fmicb.2011.00107
Estradiol-Treated Female Mice as Surrogate Hosts for Neisseria gonorrhoeae Genital Tract Infections
Abstract
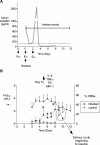
Historically, animal modeling of gonorrhea has been hampered by the exclusive adaptation of Neisseria gonorrhoeae to humans. Genital tract infection can be established in female mice that are treated with 17β-estradiol, however, and many features of experimental murine infection mimic human infection. Here we review the colonization kinetics and host response to experimental murine gonococcal infection, including mouse strain differences and evidence that IL-17 responses, toll-like receptor 4, and T regulatory cells play a role in infection. We also discuss the strengths and limitations of the mouse system and the potential of transgenic mice to circumvent host restrictions. Additionally, we review studies with genetically defined mutants that demonstrated a role for sialyltransferase and the MtrC-MtrD-MtrE active efflux pump in evading innate defenses in vivo, but not for factors hypothesized to protect against the phagocytic respiratory burst and H(2)O(2)-producing lactobacilli. Studies using estradiol-treated mice have also revealed the existence of non-host-restricted iron sources in the female genital tract and the influence of hormonal factors on colonization kinetics and selection for opacity (Opa) protein expression. Recent work by others with estradiol-treated mice that are transgenic for human carcinoembryonic adhesion molecules (CEACAMs) supports a role for Opa proteins in enhancing cellular attachment and thus reduced shedding of N. gonorrhoeae. Finally we discuss the use of the mouse model in product testing and a recently developed gonorrhea chlamydia coinfection model.
Keywords: Neisseria gonorrhoeae; Opa proteins; antimicrobial peptides; hormones; immune response; lactobacilli; mouse; neutrophils.
Figures


References
-
- Afonso S., Romagnano L., Babiarz B. (1997). The expression and function of cystatin C and cathepsin B and cathepsin L during mouse embryo implantation and placentation. Development 124, 3415–3425 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

