TonB-Dependent Transporters Expressed by Neisseria gonorrhoeae
- PMID: 21747812
- PMCID: PMC3128382
- DOI: 10.3389/fmicb.2011.00117
TonB-Dependent Transporters Expressed by Neisseria gonorrhoeae
Abstract
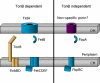
Neisseria gonorrhoeae causes the common sexually transmitted infection, gonorrhea. This microorganism is an obligate human pathogen, existing nowhere in nature except in association with humans. For growth and proliferation, N. gonorrhoeae requires iron and must acquire this nutrient from within its host. The gonococcus is well-adapted for growth in diverse niches within the human body because it expresses efficient transport systems enabling use of a diverse array of iron sources. Iron transport systems facilitating the use of transferrin, lactoferrin, and hemoglobin have two components: one TonB-dependent transporter and one lipoprotein. A single component TonB-dependent transporter also allows N. gonorrhoeae to avail itself of iron bound to heterologous siderophores produced by bacteria within the same ecological niche. Other TonB-dependent transporters are encoded by the gonococcus but have not been ascribed specific functions. The best characterized iron transport system expressed by N. gonorrhoeae enables the use of human transferrin as a sole iron source. This review summarizes the molecular mechanisms involved in gonococcal iron acquisition from human transferrin and also reviews what is currently known about the other TonB-dependent transport systems. No vaccine is available to prevent gonococcal infections and our options for treating this disease are compromised by the emergence of antibiotic resistance. Because iron transport systems are critical for the survival of the gonococcus in vivo, the surface-exposed components of these systems are attractive candidates for vaccine development or therapeutic intervention.
Keywords: Neisseria gonorrhoeae; TonB; iron; transferrin; xenosiderophores.
Figures




References
-
- Anderson J. E., Hobbs M. M., Biswas G. D., Sparling P. F. (2003). Opposing selective forces for expression of the gonococcal lactoferrin receptor. Mol. Microbiol. 48, 1325–1337 - PubMed
-
- Anderson J. E., Leonie P. A., Miller W. C., Chen C. J., Hobbs M. M., Sparling P. F. (2001). Selection for expression of the gonococcal hemoglobin receptor during menses. J. Infect. Dis. 184, 1621–1623 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

