Safety and Immunogenicity of a Human Papillomavirus Peptide Vaccine (CIGB-228) in Women with High-Grade Cervical Intraepithelial Neoplasia: First-in-Human, Proof-of-Concept Trial
- PMID: 21748025
- PMCID: PMC3118643
- DOI: 10.5402/2011/292951
Safety and Immunogenicity of a Human Papillomavirus Peptide Vaccine (CIGB-228) in Women with High-Grade Cervical Intraepithelial Neoplasia: First-in-Human, Proof-of-Concept Trial
Abstract
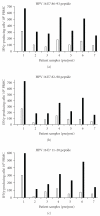
Objective. CIGB-228 is a novel therapeutic vaccine consisting of HLA-restricted HPV16 E7 epitope adjuvated with VSSP. This trial was designed to evaluate the toxicity, safety, immunogenicity, HPV clearance, and lesion regression. Methods. Seven women were entered. All were HLA-A2 positive, had biopsy-proven high-grade CIN, histologically positive for HPV16, and beared persistent postbiopsy lesions visible by digital colposcopy. HLA-A2 women with biopsy-proven high-grade CIN, HPV16-positive, and beared persistent postbiopsy lesions visible by digital colposcopy were vaccinated. One weekly injections of CIGB-228 vaccine was given for four weeks. Then, loop electrosurgical excision procedure (LEEP) of the transformation zone was performed. Study subjects were followed for 1 year after LEEP. Results. No toxicity beyond grade 1 was observed during and after the four vaccinations. Five of seven women had complete and partial regression. Cellular immune response was seen in all patients. HPV was cleared in three of the patients with complete response. Conclusion. CIGB-228 vaccination was well tolerated and capable to induce IFNγ-associated T-cell response in women with high-grade CIN. In several patients, lesion regression and HPV clearance were observed.
Figures


References
-
- Koutsky LA, Holmes KK, Critchlow CW, et al. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. The New England Journal of Medicine. 1992;327(18):1272–1278. - PubMed
-
- Muñoz N, Bosch FX, de Sanjosé S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. The New England Journal of Medicine. 2003;348(6):518–527. - PubMed
-
- Schiffman M, Kjaer SK. Chapter 2: natural history of anogenital human papillomavirus infection and neoplasia. Journal of the National Cancer Institute. Monographs. 2003;(31):14–19. - PubMed
-
- Ferenczy A, Franco E. Persistent human papillomavirus infection and cervical neoplasia. The Lancet Oncology. 2002;3(1):11–16. - PubMed
-
- Castle PE, Schiffman M, Bratti MC, et al. A population-based study of vaginal human papillomavirus infection in hysterectomized women. Journal of Infectious Diseases. 2004;190(3):458–467. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

