Aging impairs Ca2+ sensitization pathways in gallbladder smooth muscle
- PMID: 21748275
- PMCID: PMC3682072
- DOI: 10.1007/s11357-011-9285-6
Aging impairs Ca2+ sensitization pathways in gallbladder smooth muscle
Abstract
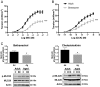
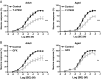
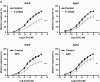
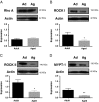
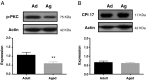
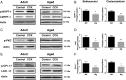
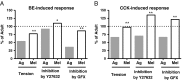
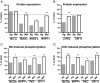
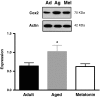
Calcium sensitization is an important physiological process in agonist-induced contraction of smooth muscle. In brief, calcium sensitization is a pathway that leads to smooth muscle contraction independently of changes in [Ca(2+)](i) by mean of inhibition of myosin light chain phosphatase. Aging has negative impacts on gallbladder contractile response due to partial impairment in calcium signaling and alterations in the contractile machinery. However, information regarding aging-induced alterations in calcium sensitization is scanty. We hypothesized that the calcium sensitization system is negatively affected by age. To investigate this, gallbladders were collected from adult (4 months old) and aged (22-24 months old) guinea pigs. To evaluate the contribution of calcium sensitization pathways we assayed the effect of the specific inhibitors Y-27632 and GF109203X on the "in vitro" isometric gallbladder contractions induced by agonist challenges. In addition, expression and phosphorylation (as activation index) of proteins participating in the calcium sensitization pathways were quantified by Western blotting. Aging reduced bethanechol- and cholecystokinin-evoked contractions, an effect associated with a reduction in MLC20 phosphorylation and in the effects of both Y-27632 and GF109203X. In addition, there was a drop in ROCK I, ROCK II, MYPT-1 and PKC expression and in the activation/phosphorylation of MYPT-1, PKC and CPI-17 in response to agonists. Interestingly, melatonin treatment for 4 weeks restored gallbladder contractile responses due to re-establishment of calcium sensitization pathways. These results demonstrate that age-related gallbladder hypocontractility is associated to alterations of calcium sensitization pathways and that melatonin treatment exerts beneficial effects in the recovery of gallbladder contractility.
Figures
Similar articles
-
Melatonin restores impaired contractility in aged guinea pig urinary bladder.J Pineal Res. 2008 May;44(4):416-25. doi: 10.1111/j.1600-079X.2007.00544.x. Epub 2008 Jan 9. J Pineal Res. 2008. PMID: 18194201
-
Myosin light chain kinase activation and calcium sensitization in smooth muscle in vivo.Am J Physiol Cell Physiol. 2008 Aug;295(2):C358-64. doi: 10.1152/ajpcell.90645.2007. Epub 2008 Jun 4. Am J Physiol Cell Physiol. 2008. PMID: 18524939 Free PMC article.
-
Age-related changes in gallbladder contractility and cytoplasmic Ca2+ concentration in the guinea pig.Am J Physiol. 1993 Apr;264(4 Pt 1):G624-9. doi: 10.1152/ajpgi.1993.264.4.G624. Am J Physiol. 1993. PMID: 8386460
-
Calcium sensitization mechanisms in detrusor smooth muscles.J Basic Clin Physiol Pharmacol. 2018 Jun 27;29(3):227-235. doi: 10.1515/jbcpp-2017-0071. J Basic Clin Physiol Pharmacol. 2018. PMID: 29306925 Review.
-
Signaling for contraction and relaxation in smooth muscle of the gut.Annu Rev Physiol. 2006;68:345-74. doi: 10.1146/annurev.physiol.68.040504.094707. Annu Rev Physiol. 2006. PMID: 16460276 Review.
Cited by
-
Aging-associated oxidative stress leads to decrease in IAS tone via RhoA/ROCK downregulation.Am J Physiol Gastrointest Liver Physiol. 2014 Jun 1;306(11):G983-91. doi: 10.1152/ajpgi.00087.2014. Epub 2014 Apr 17. Am J Physiol Gastrointest Liver Physiol. 2014. PMID: 24742984 Free PMC article.
-
Modulation of Diabetes-Induced Oxidative Stress, Apoptosis, and Ca2+ Entry Through TRPM2 and TRPV1 Channels in Dorsal Root Ganglion and Hippocampus of Diabetic Rats by Melatonin and Selenium.Mol Neurobiol. 2017 Apr;54(3):2345-2360. doi: 10.1007/s12035-016-9727-3. Epub 2016 Mar 9. Mol Neurobiol. 2017. PMID: 26957303
-
Disruption of gallbladder smooth muscle function is an early feature in the development of cholesterol gallstone disease.Neurogastroenterol Motil. 2012 Jul;24(7):e313-24. doi: 10.1111/j.1365-2982.2012.01935.x. Epub 2012 May 24. Neurogastroenterol Motil. 2012. PMID: 22621672 Free PMC article.
References
-
- Camilleri M, Lee JS, Viramontes B, Bharucha AE, Tangalos EG. Insights into the pathophysiology and mechanisms of constipation, irritable bowel syndrome, and diverticulosis in older people. J Am Geriatr Soc. 2000;48:1142–1150. - PubMed
-
- Chiba Y, Takada Y, Miyamoto S, MitsuiSaito M, Karaki H, Misawa M. Augmented acetylcholine-induced, Rho-mediated Ca2+ sensitization of bronchial smooth muscle contraction in antigen-induced airway hyperresponsive rats. Br J Pharmacol. 1999;127:597–600. doi: 10.1038/sj.bjp.0702585. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
