Characterization of Kupffer cells in livers of developing mice
- PMID: 21749715
- PMCID: PMC3148529
- DOI: 10.1186/1476-5926-10-2
Characterization of Kupffer cells in livers of developing mice
Abstract
Background: Kupffer cells are well known macrophages of the liver, however, the developmental characteristics of Kupffer cells in mice are not well understood. To clarify this matter, the characteristics of Kupffer macrophages in normal developing mouse liver were studied using light microscopy and immunocytochemistry.
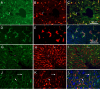
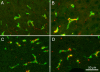
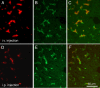
Methods: Sections of liver tissue from early postnatal mice were prepared using immunocytochemical techniques. The Kupffer cells were identified by their immunoreactivity to the F4/80 antibody, whereas endothelial cells were labelled with the CD-34 antibody. In addition, Kupffer cells and endothelial cells were labelled by systemically injected fluorescently labelled latex microspheres. Tissue slices were examined by fluorescence microscopy.
Results: Intravenous or intraperitonal injections of microspheres yielded similar patterns of liver cell labelling. The F4/80 positive Kupffer cells were labelled with both large (0.2 μm) and small (0.02 μm) diameter microspheres, while endothelial cells were labelled only with the smaller diameter microspheres. Microsphere labelling of Kupffer cells appeared stable for at least 6 weeks. Cells immunoreactive for F4/80 were identified as early as postnatal day 0, and these cells also displayed uptake of microspheres. Numbers of F4/80 Kupffer cells, relative to numbers of albumin positive hepatocytes, did not show a significant trend over the first 2 postnatal weeks.
Conclusions: Kupffer cells of the developing mouse liver appear quite similar to those of other mammalian species, confirming that the mouse presents a useful animal model for studies of liver macrophage developmental structure and function.
Figures



References
-
- Wisse E. An ultrastructural characterization of the endothelial cell in the rat liver sinusoid under normal and various experimental conditions, as a contribution to the distinction between endothelial and Kupffer cells. J Ultrastruct Res. 1972;38:528–562. doi: 10.1016/0022-5320(72)90089-5. - DOI - PubMed
-
- Fahimi HD. In: The Liver: Biology and Pathobiology. Arias IM, Popper H, Schachter D, Shafritz DA, editor. Raven Press New York; 1982. Sinusoidal endothelial cells and perisinusoidal fat-storing cells: structure and function; pp. 495–506.
