Magnetic iron oxide nanoparticles: synthesis and surface functionalization strategies
- PMID: 21749733
- PMCID: PMC3244954
- DOI: 10.1007/s11671-008-9174-9
Magnetic iron oxide nanoparticles: synthesis and surface functionalization strategies
Abstract
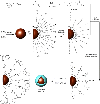
Surface functionalized magnetic iron oxide nanoparticles (NPs) are a kind of novel functional materials, which have been widely used in the biotechnology and catalysis. This review focuses on the recent development and various strategies in preparation, structure, and magnetic properties of naked and surface functionalized iron oxide NPs and their corresponding application briefly. In order to implement the practical application, the particles must have combined properties of high magnetic saturation, stability, biocompatibility, and interactive functions at the surface. Moreover, the surface of iron oxide NPs could be modified by organic materials or inorganic materials, such as polymers, biomolecules, silica, metals, etc. The problems and major challenges, along with the directions for the synthesis and surface functionalization of iron oxide NPs, are considered. Finally, some future trends and prospective in these research areas are also discussed.
Figures




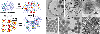
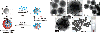

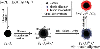
References
-
- LaConte L, Nitin N, Bao G. Mater. Today. 2005. p. 32. - DOI
-
- Patel D, Moon JY, Chang Y, Kim TJ, Lee GH. Colloid Surf. A. 2008. p. 91. COI number [1:CAS:528:DC%2BD1cXkvF2n] - DOI
-
- Mornet S, Vasseur S, Grasset F, Veverka P, Goglio G, Demourgues A, Prog. 2006. p. 237. COI number [1:CAS:528:DC%2BD28Xls1Gmsrc%3D] - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

