Same-session functional assessment of rat retina and brain with manganese-enhanced MRI
- PMID: 21749922
- PMCID: PMC3787253
- DOI: 10.1016/j.neuroimage.2011.06.062
Same-session functional assessment of rat retina and brain with manganese-enhanced MRI
Abstract
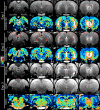
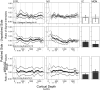
Manganese-enhanced MRI (MEMRI) is a powerful non-invasive approach for objectively measuring either retina or binocular visual brain activity in vivo. In this study, we investigated the sensitivity of MEMRI to monocular stimulation using a new protocol for providing within-subject functional comparisons in the retina and brain in the same scanning session. Adult Sprague Dawley or Long-Evans rats had one eye covered with an opaque patch. After intraperitoneal Mn(2+) administration on the following day, rats underwent visual stimulation for 8h. Animals were then anesthetized, and the brain and each eye examined by MEMRI. Function was assessed through pairwise comparisons of the patched (dark-adapted) versus unpatched (light-exposed) eyes, and of differentially-stimulated brain structures - the dorsal lateral geniculate nucleus, superior colliculus, and visual cortical regions - contralateral to the patched versus unpatched eye. As expected, Mn(2+) uptake was greater in the outer retina of dark-adapted, relative to light-exposed, eyes (P<0.05). Contralateral to the unpatched eye, significantly more Mn(2+) uptake was found throughout the visual brain regions than in the corresponding structures contralateral to the patched eye (P<0.05). Notably, this regional pattern of activity corresponded well to previous work with monocular stimulation. No stimulation-dependent differences in Mn(2+) uptake were observed in negative control brain regions (P>0.05). Post-hoc assessment of functional data by animal age and strain revealed no significant effects. These results demonstrate, for the first time, the acquisition of functional MRI data from the eye and visual brain regions in a single scanning session.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures




References
-
- Alvestad S, Goa PE, Qu H, Risa Ø, Brekken C, Sonnewald U, Haraldseth O, Hammer J, Ottersen OP, HåBerg A. In vivo mapping of temporospatial changes in manganese enhancement in rat brain during epileptogenesis. Neuro-Image. 2007;38:57–66. - PubMed
-
- Angenstein F, Niessen HG, Goldschmidt J, Lison H, Altrock WD, Gundelfinger ED, Scheich H. Manganese-enhanced MRI reveals structural and functional changes in the cortex of bassoon mutant mice. Cerebral Cortex. 2007;17:28–36. - PubMed
-
- Batipps M, Miyaoka M, Shinohara M, Sokoloff L, Kennedy C. Comparative rates of local cerebral glucose utilization in the visual system of conscious albino and pigmented rats. Neurology. 1981;31:58–62. - PubMed
-
- Berkowitz BA, Roberts R, Goebel DJ, Luan H. Noninvasive and simultaneous imaging of layer-specific retinal functional adaptation by manganese-enhanced MRI. Investigative Ophthalmology and Visual Science. 2006;47(6):2668–2674. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

