Prolonged intracerebral convection-enhanced delivery of topotecan with a subcutaneously implantable infusion pump
- PMID: 21750007
- PMCID: PMC3145467
- DOI: 10.1093/neuonc/nor051
Prolonged intracerebral convection-enhanced delivery of topotecan with a subcutaneously implantable infusion pump
Abstract
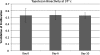
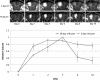
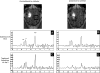
Intracerebral convection-enhanced delivery (CED) of chemotherapeutic agents currently requires an externalized catheter and infusion system, which limits its duration because of the need for hospitalization and the risk of infection. To evaluate the feasibility of prolonged topotecan administration by CED in a large animal brain with the use of a subcutaneous implantable pump. Medtronic Synchromed-II pumps were implanted subcutaneously for intracerebral CED in pigs. Gadodiamide (28.7 mg/mL), with or without topotecan (136 μM), was infused at 0.7 mL/24 h for 3 or 10 days. Pigs underwent magnetic resonance imaging before and at 6 times points after surgery. Enhancement and FLAIR+ volumes were calculated in a semi-automated fashion. Magnetic resonance spectroscopy-based topotecan signature was also investigated. Brain histology was analyzed by hematoxylin and eosin staining and with immunoperoxidase for a microglial antigen. CED of topotecan/gadolinium was well tolerated in all cases (n = 6). Maximum enhancement volume was reached at day 3 and remained stable if CED was continued for 10 days, but it decreased if CED was stopped at day 3. Magnetic resonance spectroscopy revealed a decrease in parenchymal metabolites in the presence of topotecan. Similarly, the combination of topotecan and gadolinium infusion led to a FLAIR+ volume that tended to be larger than that seen after the infusion of gadolinium alone. Histological analysis of the brains showed an area of macrophage infiltrate in the ipsilateral white matter upon infusion with topotecan/gadolinium. Intracerebral topotecan CED is well tolerated in a large animal brain for up to 10 days. Intracerebral long-term CED can be achieved with a subcutaneously implanted pump and provides a stable volume of distribution. This work constitutes a proof of principle for the safety and feasibility for prolonged CED, providing a means of continuous local drug delivery that is accessible to the practicing neuro-oncologist.
Figures


References
-
- Lesniak MS, Brem H. Targeted therapy for brain tumours. Nat Rev Drug Discov. 2004;3(6):499–508. doi:10.1038/nrd1414. - DOI - PubMed
-
- Barker FG, 2nd, Chang SM, Gutin PH, et al. Survival and functional status after resection of recurrent glioblastoma multiforme. Neurosurgery. 1998;42(4):709–720. discussion 720–703 doi:10.1097/00006123-199804000-00013. - DOI - PubMed
-
- Laske DW, Youle RJ, Oldfield EH. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat Med. 1997;3(12):1362–1368. doi:10.1038/nm1297-1362. - DOI - PubMed
-
- Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA. 1994;91(6):2076–2080. doi:10.1073/pnas.91.6.2076. - DOI - PMC - PubMed
-
- Lopez KA, Waziri AE, Canoll PD, Bruce JN. Convection-enhanced delivery in the treatment of malignant glioma. Neurol Res. 2006;28(5):542–548. doi:10.1179/016164106X116836. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

