Signaling in H2O2-induced increase in cell proliferation in Barrett's esophageal adenocarcinoma cells
- PMID: 21750116
- PMCID: PMC3186290
- DOI: 10.1124/jpet.111.182352
Signaling in H2O2-induced increase in cell proliferation in Barrett's esophageal adenocarcinoma cells
Abstract
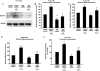
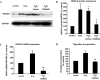
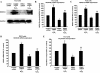
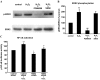
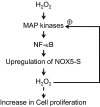
Mechanisms whereby acid reflux may accelerate the progression from Barrett's esophagus (BE) to esophageal adenocarcinoma (EA) are not fully understood. We have previously shown that NADPH oxidase NOX5-S generates reactive oxygen species (ROS) when Barrett's metaplastic cells are exposed to acid. Besides metaplastic cells, other H(2)O(2)-producing cells (e.g., inflammatory cells) present in BE mucosa may produce additional ROS, which may also affect metaplastic cells contributing to esophageal tumorigenesis. In this study, we investigate whether exogenous H(2)O(2) stimulates cell proliferation by increasing NOX5-S expression. Low dose (10(-13) M) of H(2)O(2) significantly increased thymidine incorporation, NOX5-S mRNA, and protein expression in a Barrett's EA cell line FLO. H(2)O(2)-induced increase in NOX5-S expression was significantly inhibited by knockdown of nuclear factor (NF)-κB1 p50 with p50 small interfering RNA (siRNA) in EA cell lines FLO and OE33. H(2)O(2) significantly increased p65 phosphorylation and the luciferase activity in FLO cells transfected with a NF-κB activation reporter plasmid pNF-κB-Luc. H(2)O(2)-induced increase in luciferase activity in FLO cells was significantly decreased by knockdown of extracellular signal-regulated kinase 2 (ERK2) mitogen-activated protein kinase (MAPK). Overexpression of p50 and p65 remarkably increased the luciferase activity in FLO cells transfected with a NOX5-S reporter plasmid NOX5-LP. In addition, H(2)O(2)-induced thymidine incorporation in FLO cells was significantly decreased by the MAPK kinase 1/2 inhibitor 2'-amino-3'methoxyflavone (PD98059) and ERK2 siRNA but not by ERK1 siRNA. Likewise, H(2)O(2)-induced increase in NOX5-S expression was significantly decreased by ERK2 siRNA in FLO and OE33 cells. We conclude that a low dose of H(2)O(2) increases cell proliferation. H(2)O(2)-induced increase in cell proliferation may depend on sequential activation of ERK2 MAPK, NF-κB1 p50, and NOX5-S.
Figures


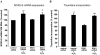
Similar articles
-
NADPH oxidase NOX5-S mediates acid-induced cyclooxygenase-2 expression via activation of NF-kappaB in Barrett's esophageal adenocarcinoma cells.J Biol Chem. 2007 Jun 1;282(22):16244-55. doi: 10.1074/jbc.M700297200. Epub 2007 Apr 2. J Biol Chem. 2007. PMID: 17403674
-
NADPH oxidase NOX5-S and nuclear factor κB1 mediate acid-induced microsomal prostaglandin E synthase-1 expression in Barrett's esophageal adenocarcinoma cells.Mol Pharmacol. 2013 May;83(5):978-90. doi: 10.1124/mol.112.083287. Epub 2013 Feb 25. Mol Pharmacol. 2013. PMID: 23439561 Free PMC article.
-
Role of intracellular calcium and NADPH oxidase NOX5-S in acid-induced DNA damage in Barrett's cells and Barrett's esophageal adenocarcinoma cells.Am J Physiol Gastrointest Liver Physiol. 2014 May 15;306(10):G863-72. doi: 10.1152/ajpgi.00321.2013. Epub 2014 Apr 3. Am J Physiol Gastrointest Liver Physiol. 2014. PMID: 24699332 Free PMC article.
-
Overview of major molecular alterations during progression from Barrett's esophagus to esophageal adenocarcinoma.Ann N Y Acad Sci. 2016 Oct;1381(1):74-91. doi: 10.1111/nyas.13134. Epub 2016 Jul 14. Ann N Y Acad Sci. 2016. PMID: 27415609 Review.
-
Barrett's oesophagus and oesophageal adenocarcinoma: how does acid interfere with cell proliferation and differentiation?Gut. 2005 Mar;54 Suppl 1(Suppl 1):i21-6. doi: 10.1136/gut.2004.041558. Gut. 2005. PMID: 15711004 Free PMC article. Review.
Cited by
-
ROS-induced cell cycle arrest as a mechanism of resistance in polyaneuploid cancer cells (PACCs).Prog Biophys Mol Biol. 2021 Oct;165:3-7. doi: 10.1016/j.pbiomolbio.2021.05.002. Epub 2021 May 12. Prog Biophys Mol Biol. 2021. PMID: 33991583 Free PMC article.
-
Evolution of the ferric reductase domain (FRD) superfamily: modularity, functional diversification, and signature motifs.PLoS One. 2013;8(3):e58126. doi: 10.1371/journal.pone.0058126. Epub 2013 Mar 7. PLoS One. 2013. PMID: 23505460 Free PMC article.
-
Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system.Circ Res. 2012 May 11;110(10):1364-90. doi: 10.1161/CIRCRESAHA.111.243972. Circ Res. 2012. PMID: 22581922 Free PMC article. Review.
-
Characterization of NADPH oxidase 5 expression in human tumors and tumor cell lines with a novel mouse monoclonal antibody.Free Radic Biol Med. 2013 Dec;65:497-508. doi: 10.1016/j.freeradbiomed.2013.07.005. Epub 2013 Jul 11. Free Radic Biol Med. 2013. PMID: 23851018 Free PMC article.
-
UGT2B17 and miR-224 contribute to hormone dependency trends in adenocarcinoma and squamous cell carcinoma of esophagus.Biosci Rep. 2019 Jul 5;39(7):BSR20190472. doi: 10.1042/BSR20190472. Print 2019 Jul 31. Biosci Rep. 2019. PMID: 31164411 Free PMC article.
References
-
- Bánfi B, Maturana A, Jaconi S, Arnaudeau S, Laforge T, Sinha B, Ligeti E, Demaurex N, Krause KH. (2000) A mammalian H+ channel generated through alternative splicing of the NADPH oxidase homolog NOH-1. Science 287:138–142 - PubMed
-
- Blot WJ, McLaughlin JK. (1999) The changing epidemiology of esophageal cancer. Semin Oncol 26 (5 Suppl 15):2–8 - PubMed
-
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 - PubMed
-
- Cao W, Sohn UD, Bitar KN, Behar J, Biancani P, Harnett KM. (2003) MAPK mediates PKC-dependent contraction of cat esophageal and lower esophageal sphincter circular smooth muscle. Am J Physiol Gastrointest Liver Physiol 285:G86–G95 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

