Effect of administration of moxifloxacin plus rifampin against Mycobacterium tuberculosis for 7 of 7 days versus 5 of 7 days in an in vitro pharmacodynamic system
- PMID: 21750119
- PMCID: PMC3132875
- DOI: 10.1128/mBio.00108-11
Effect of administration of moxifloxacin plus rifampin against Mycobacterium tuberculosis for 7 of 7 days versus 5 of 7 days in an in vitro pharmacodynamic system
Abstract
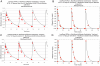
Some trials administered antituberculosis agents for 5 of 7 days (5/7-day regimen) to optimize adherence. Since moxifloxacin has a longer half-life than rifampin, rifampin concentrations are <1% of the maximum concentration in serum (C(max)) on day 6 and nondetectable on day 7, while concentrations of moxifloxacin remain and are able to induce error-prone replication. We determined if functional moxifloxacin monotherapy for 24 h/week caused resistance. In in vitro pharmacodynamic experiments, Mycobacterium tuberculosis was treated with mean area under the concentration-time curve (AUC) exposures for moxifloxacin and rifampin of 400 and 600 mg/kg/day and exposures equal to 1 standard deviation (SD) above and below the mean values. The drugs were administered on schedules of 7/7 days and 5/7 days. Over the 28-day experiments, bacteria were plated onto antibiotic-free agar to determine the effects of exposure and schedule on the total population. MICs were checked for emergence of resistance. At days 7 and 14, there was a 0.56- to 1.22-log(10)-CFU/ml greater cell kill with the 7/7-day regimen versus the 5/7-day regimen (low exposure). This difference was not seen for the larger exposures at day 21. At day 23, the low-exposure 5/7-day arm had breakthrough resistance, with the total count increasing to >2 log(10) CFU/ml above the low-exposure 7/7-day arm. Pharmacokinetic mismatching of drugs in the therapy of tuberculosis may result in emergence of resistance when a drug holiday is imposed during which there is functional monotherapy and where the remaining agent induces error-prone replication. This is particularly true for the portion of the population where the clearance is higher (1 SD above the mean).
Importance: Directly observed therapy is a cornerstone of treatment of Mycobacterium tuberculosis. Patients are often given a drug holiday to facilitate the direct observation of therapy. With rifampin and moxifloxacin, there is a discordance between the half-lives of these agents (1.9 versus 6.5 h when employed in combination). In addition, moxifloxacin induces error-prone replication in Mycobacterium tuberculosis. In this experiment, we demonstrate that the drug holiday (5 of 7 days of therapy [5/7-day regimen]) allows the emergence of resistance to moxifloxacin, which was not seen with 7/7-day therapy. If drug holidays are used, it is imperative to better match pharmacokinetics to minimize the risk of emergence of resistance.
Figures

References
-
- Dorman SE, et al. 2009. Substitution of moxifloxacin for isoniazid during intensive phase treatment of pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 180:273–280 - PubMed
-
- Weiner M, et al. 2004. Pharmacokinetics of rifapentine at 600, 900, and 1,200 mg during once-weekly tuberculosis therapy. Am. J. Respir. Crit. Care Med. 169:1191–1197 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
