MMP9 cleavage of the β4 integrin ectodomain leads to recurrent epithelial erosions in mice
- PMID: 21750188
- PMCID: PMC3138707
- DOI: 10.1242/jcs.085480
MMP9 cleavage of the β4 integrin ectodomain leads to recurrent epithelial erosions in mice
Abstract
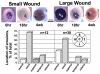
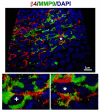
Integrin α6β4 is an integral membrane protein within hemidesmosomes and it mediates adhesion of epithelial cells to their underlying basement membrane. During wound healing, disassembly of hemidesmosomes must occur for sheet movement-mediated cell migration. The mechanisms of disassembly and reassembly of hemidesmosomes are not fully understood. The current study was initiated to understand the underlying cause of recurrent corneal erosions in the mouse. Here, we show that in vivo: (1) MMP9 levels are elevated and β4 integrin is partially cleaved in epithelial cell extracts derived from debridement wounded corneas; (2) the β4 ectodomain is missing from sites where erosions develop; and (3) β4 cleavage can be reduced by inhibiting MMP activity. Although β4, α3 and β1 integrins were all cleaved by several MMPs, only MMP9 was elevated in cell extracts derived from corneas with erosions. Coimmunoprecipitation studies showed that β4 integrin associates with MMP9, and protein clustering during immunoprecipitation induced proteolytic cleavage of the β4 integrin extracellular domain, generating a 100 kDa β4 integrin cytoplasmic domain fragment. Confocal imaging with three-dimensional reconstruction showed that MMP9 localizes at erosion sites in vivo where the ectodomain of β4 integrin is reduced or absent. MMP activation experiments using cultured corneal and epidermal keratinocytes showed reduced levels of α6β4 and β1 integrins within 20 minutes of phorbol ester treatment. This report is the first to show that β4 integrin associates with MMP9 and that its ectodomain is a target for cleavage by MMP9 in vivo under pathological conditions.
Figures





References
-
- Borradori L., Sonnenberg A. (1996). Hemidesmosomes: roles in adhesion, signaling and human diseases. Curr. Opin. Cell Biol. 8, 647-656 - PubMed
-
- Choi W. S., Jeon O. H., Kim D. S. (2010). CD40 ligand shedding is regulated by interaction between matrix metalloproteinase-2 and platelet integrin αIIbβ3. J. Thromb. Haemost. 8, 1364-1371 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

