Stimulated association of STIM1 and Orai1 is regulated by the balance of PtdIns(4,5)P₂ between distinct membrane pools
- PMID: 21750194
- PMCID: PMC3138702
- DOI: 10.1242/jcs.084178
Stimulated association of STIM1 and Orai1 is regulated by the balance of PtdIns(4,5)P₂ between distinct membrane pools
Abstract
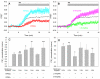
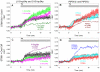
We have previously shown that PIP5KIβ and PIP5KIγ generate functionally distinct pools of phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P(2)] important for antigen-stimulated Ca(2+) entry in mast cells. In the present study, we find that association of the endoplasmic reticulum (ER) Ca(2+) sensor, STIM1, and the store-operated Ca(2+) channel, Orai1, stimulated by thapsigargin-mediated ER store depletion, is enhanced by overexpression of PIP5KIβ and inhibited by overexpression of PIP5KIγ. These different PIP5KI isoforms cause differential enhancement of PtdIns(4,5)P(2) in detergent-resistant membrane (DRM) fractions, which comprise ordered lipid regions, and detergent-solubilized membrane (DSM) fractions, which comprise disordered lipid regions. Consistent with these results, the inositol 5-phosphatase L10-Inp54p, which is targeted to ordered lipids, decreases PtdIns(4,5)P(2) in the DRM fraction and inhibits thapsigargin-stimulated STIM1-Orai1 association and store-operated Ca(2+) entry, whereas the inositol 5-phosphatase S15-Inp54p, which is targeted to disordered lipids, decreases PtdIns(4,5)P(2) in the DSM fraction and enhances STIM1-Orai1 association. Removal of either the STIM1 C-terminal polylysine sequence (amino acids 677-685) or an N-terminal polyarginine sequence in Orai1 (amino acids 28-33) eliminates this differential sensitivity of STIM1-Orai1 association to PtdIns(4,5)P(2) in the distinctive membrane domains. Our results are consistent with a model of PtdIns(4,5)P(2) balance, in which store-depletion-stimulated STIM1-Orai1 association is positively regulated by the ordered lipid pool of PtdIns(4,5)P(2) and negatively regulated by PtdIns(4,5)P(2) in disordered lipid domains.
Figures



References
-
- Alicia S., Angélica Z., Carlos S., Alfonso S., Vaca L. (2008). STIM1 converts TRPC1 from a receptor-operated to a store-operated channel: moving TRPC1 in and out of lipid rafts. Cell Calcium 44, 479-491 - PubMed
-
- Bauer M. C., O'Connell D., Cahill D. J., Linse S. (2008). Calmodulin binding to the polybasic C-termini of STIM proteins involved in store-operated calcium entry. Biochemistry 47, 6089-6091 - PubMed
-
- Broad L. M., Braun F. J., Lievremont J. P., Bird G. S., Kurosaki T., Putney J. W. (2001). Role of the phospholipase C-inositol 1,4,5-trisphosphate pathway in calcium release-activated calcium current and capacitative calcium entry. J. Biol. Chem. 276, 15945-15952 - PubMed
-
- Brown D. A. (2006). Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology 21, 430-439 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

