Decreased proteolytic activity of the mitochondrial amyloid-β degrading enzyme, PreP peptidasome, in Alzheimer's disease brain mitochondria
- PMID: 21750375
- PMCID: PMC3381900
- DOI: 10.3233/JAD-2011-101716
Decreased proteolytic activity of the mitochondrial amyloid-β degrading enzyme, PreP peptidasome, in Alzheimer's disease brain mitochondria
Abstract
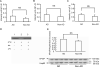
Accumulation of amyloid-β peptide (Aβ), the neurotoxic peptide implicated in the pathogenesis of Alzheimer's disease (AD), has been shown in brain mitochondria of AD patients and of AD transgenic mouse models. The presence of Aβ in mitochondria leads to free radical generation and neuronal stress. Recently, we identified the presequence protease, PreP, localized in the mitochondrial matrix in mammalian mitochondria as the novel mitochondrial Aβ-degrading enzyme. In the present study, we examined PreP activity in the mitochondrial matrix of the human brain's temporal lobe, an area of the brain highly susceptible to Aβ accumulation and reactive oxygen species (ROS) production. We found significantly lower hPreP activity in AD brains compared with non-AD age-matched controls. By contrast, in the cerebellum, a brain region typically spared from Aβ accumulation, there was no significant difference in hPreP activity when comparing AD samples to non-AD controls. We also found significantly reduced PreP activity in the mitochondrial matrix of AD transgenic mouse brains (Tg mAβPP and Tg mAβPP/ABAD) when compared to non-transgenic aged-matched mice. Furthermore, mitochondrial fractions isolated from AD brains and Tg mAβPP mice had higher levels of 4-hydroxynonenal, an oxidative product, as compared with those from non-AD and nonTg mice. Accordingly, activity of cytochrome c oxidase was significantly reduced in the AD mitochondria. These findings suggest that decreased PreP proteolytic activity, possibly due to enhanced ROS production, contributes to Aβ accumulation in mitochondria leading to the mitochondrial toxicity and neuronal death that is exacerbated in AD. Clearance of mitochondrial Aβ by PreP may thus be of importance in the pathology of AD.
Figures



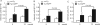

References
-
- Selkoe DJ. Translating cell biology into therapeutic advances in Alzheimer's disease. Nature. 1999;399:A23–A31. - PubMed
-
- Soderberg L, Bogdanovic N, Axelsson B, Winblad B, Naslund J, Tjernberg LO. Analysis of single Alzheimer solid plaque cores by laser capture microscopy and nano-electrospray/tandem mass spectrometry. Biochemistry. 2006;45:9849–9856. - PubMed
-
- Gouras GK, Almeida CG, Takahashi RH. Intraneuronal Abeta accumulation and origin of plaques in Alzheimer's disease. Neurobiol Aging. 2005;26:1235–1244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

