A prospective randomized double-blinded pilot study to examine the effect of botulinum toxin type A injection versus Lidocaine/Depomedrol injection on residual and phantom limb pain: initial report
- PMID: 21750460
- PMCID: PMC3845354
- DOI: 10.1097/AJP.0b013e3182264fe9
A prospective randomized double-blinded pilot study to examine the effect of botulinum toxin type A injection versus Lidocaine/Depomedrol injection on residual and phantom limb pain: initial report
Abstract
Objective: Botulinum toxin type A (Botox) injection has been used to manage pain. However, it remains to be proved whether Botox injection is effective to relieve residual limb pain (RLP) and phantom limb pain (PLP).
Design: Randomized, double-blinded pilot study.
Setting: Medical College and an outpatient clinic in Department of Physical Medicine and Rehabilitation.
Participants: Amputees (n=14) with intractable RLP and/or PLP who failed in the conventional treatments.
Interventions: Study amputees were randomized to receive 1 Botox injection versus the combination of Lidocaine and Depomedrol injection. Each patient was evaluated at baseline and every month after the injection for 6 months.
Main outcome measure: The changes of RLP and PLP as recorded by VAS, and the changes of the pressure pain tolerance as determined by a pressure algometer.
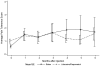
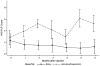
Results: All patients completed the protocol treatment without acute side effects, and monthly assessments of RLP, PLP, and pain tolerance after the treatment. The time trend in the outcomes was modeled as an immediate change owing to the treatment followed by a linear tread afterward. Repeated measures were incorporated using mixed effects modeling. We found that both Botox and Lidocaine/Depomedrol injections resulted in immediate improvements of RLP (Botox: P=0.002; Lidocaine/Depomedrol: P=0.06) and pain tolerance (Botox: P=0.01; Lidocaine/Depomedrol: P=0.07). The treatment effect lasted for 6 months in both groups. The patients who received Botox injection had higher starting pain than those who received Lidocaine/Depomedrol injection (P=0.07). However, there were no statistical differences in RLP and pain tolerance between these 2 groups. In addition, no improvement of PLP was observed after Botox or Lidocaine/Depomedrol injection.
Conclusions: Both Botox and Lidocaine/Depomedrol injections resulted in immediate improvement of RLP (not PLP) and pain tolerance, which lasted for 6 months in amputees who failed in conventional treatments.
Conflict of interest statement
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the author(s) or upon any organization with which the author(s) is/are associated.
The authors declare no conflict of interest.
Figures

References
-
- Sherman RA. Phantom Pain. New York: Plenum Press; 1997.
-
- Nikolajsen L, Jensen TS. Phantom limb pain. Br J Anesth. 2001;87:107–116. - PubMed
-
- Flor H, Birbaumer N, Sherman R. Phantom limb pain. Pain: Clinical Update. International Association for the Study of Pain. 2000;VIII(3):1–4.
-
- Rhamachandran VS, Hirstein W. The perception of phantom limbs. The D.O. Hebb Lecture. Brain. 1998;121:1603–1630. - PubMed
-
- Katz J. Prevention of phantom limb pain by regional anesthesia. Lancet. 1997;349:519–520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

