Calcium modulates force sensing by the von Willebrand factor A2 domain
- PMID: 21750539
- PMCID: PMC3144584
- DOI: 10.1038/ncomms1385
Calcium modulates force sensing by the von Willebrand factor A2 domain
Abstract
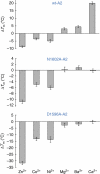
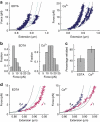
von Willebrand factor (VWF) multimers mediate primary adhesion and aggregation of platelets. VWF potency critically depends on multimer size, which is regulated by a feedback mechanism involving shear-induced unfolding of the VWF-A2 domain and cleavage by the metalloprotease ADAMTS-13. Here we report crystallographic and single-molecule optical tweezers data on VWF-A2 providing mechanistic insight into calcium-mediated stabilization of the native conformation that protects A2 from cleavage by ADAMTS-13. Unfolding of A2 requires higher forces when calcium is present and primarily proceeds through a mechanically stable intermediate with non-native calcium coordination. Calcium further accelerates refolding markedly, in particular, under applied load. We propose that calcium improves force sensing by allowing reversible force switching under physiologically relevant hydrodynamic conditions. Our data show for the first time the relevance of metal coordination for mechanical properties of a protein involved in mechanosensing.
Figures




References
-
- Sadler J. E. Biochemistry and genetics of von Willebrand factor. Annu. Rev. Biochem. 67, 395–424 (1998). - PubMed
-
- Ruggeri Z. M. Von Willebrand factor. Curr. Opin. Hematol. 10, 142–149 (2003). - PubMed
-
- Wagner D. D. Cell biology of von Willebrand factor. Annu. Rev. Cell Biol. 6, 217–246 (1990). - PubMed
-
- Furlan M., Robles R. & Lamie B. Partial purification and characterization of a protease from human plasma cleaving von Willebrand factor to fragments produced by in vivo proteolysis. Blood 87, 4223–4234 (1996). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

