Mechanism of 150-cavity formation in influenza neuraminidase
- PMID: 21750542
- PMCID: PMC3144582
- DOI: 10.1038/ncomms1390
Mechanism of 150-cavity formation in influenza neuraminidase
Abstract
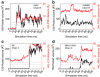
The recently discovered 150-cavity in the active site of group-1 influenza A neuraminidase (NA) proteins provides a target for rational structure-based drug development to counter the increasing frequency of antiviral resistance in influenza. Surprisingly, the 2009 H1N1 pandemic virus (09N1) neuraminidase was crystalized without the 150-cavity characteristic of group-1 NAs. Here we demonstrate, through a total sum of 1.6 μs of biophysical simulations, that 09N1 NA exists in solution preferentially with an open 150-cavity. Comparison with simulations using avian N1, human N2 and 09N1 with a I149V mutation and an extensive bioinformatics analysis suggests that the conservation of a key salt bridge is crucial in the stabilization of the 150-cavity across both subtypes. This result provides an atomic-level structural understanding of the recent finding that antiviral compounds designed to take advantage of contacts in the 150-cavity can inactivate both 2009 H1N1 pandemic and avian H5N1 viruses.
Figures

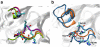
References
-
- Russell R. J. et al.. The structure of H5N1 avian influenza neuraminidase suggests new opportunities for drug design. Nature 443, 45–49 (2006). - PubMed
-
- von Itzstein M. The war against influenza: discovery and development of sialidase inhibitors. Nat. Rev. Drug Discov. 6, 967–974 (2007). - PubMed
-
- Dharan N. J. et al.. Infections with oseltamivir-resistant influenza A(H1N1) virus in the United States. JAMA 301, 1034–1041 (2009). - PubMed
-
- Li Q. et al.. The 2009 pandemic H1N1 neuraminidase N1 lacks the 150-cavity in its active site. Nat. Struct. Mol. Biol. 17, 1266–1268 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

