A randomised, phase II study of intetumumab, an anti-αv-integrin mAb, alone and with dacarbazine in stage IV melanoma
- PMID: 21750555
- PMCID: PMC3172894
- DOI: 10.1038/bjc.2011.183
A randomised, phase II study of intetumumab, an anti-αv-integrin mAb, alone and with dacarbazine in stage IV melanoma
Abstract
Background: α(v) integrins are involved in angiogenesis and melanoma tumourigenesis. Intetumumab (CNTO 95) is a fully human anti-α(v)-integrin monoclonal antibody.
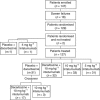
Methods: In a multicentre, randomised, phase II study, stage IV melanoma patients were randomised 1:1:1:1 to 1000 mg m(-2) dacarbazine+placebo (n=32), 1000 mg m(-2) dacarbazine+10 mg kg(-1) intetumumab (n=32), 10 mg kg(-1) intetumumab (n=33), or 5 mg kg(-1) intetumumab (n=32) q3w. The primary endpoint was progression-free survival (PFS). Secondary endpoints included overall survival (OS), objective response rate (ORR), adverse events, and pharmacokinetics.
Results: No statistically significant differences in efficacy were observed between groups. In the dacarbazine+placebo, dacarbazine+intetumumab, 10 mg kg(-1) intetumumab, and 5 mg kg(-1) intetumumab groups, median PFS was 1.8, 2.5, 1.4, and 1.4 months; median OS was 8, 11, 15, and 9.8 months; and ORR of complete+partial response was 10, 3, 6, and 0%. Nonlinear intetumumab pharmacokinetics and potential intetumumab-dacarbazine interactions were observed. Transient, asymptomatic, nonrecurring, grade 1-2, uveitic reactions that resolved spontaneously or with topical steroids were seen in 22-30% of intetumumab-treated patients. Low-grade infusion-reaction symptoms (headache, fatigue, nausea, vomiting, fever, chills) were observed, as expected, in 16-73% of dacarbazine-treated patients. No intetumumab-related myelosuppression, laboratory/electrocardiogram abnormalities, or deaths occurred.
Conclusion: With its favourable safety profile and a nonsignificant trend towards improved OS, intetumumab merits further investigation in advanced melanoma.
Conflict of interest statement
O’Day, Pavlick, Loquai, Lawson, Gutzmer, Richards, Schadendorf, Thompson, Gonzalez, Trefzer, Mohr, Ottensmeier, Chao, and Gore received institutional research grants from Centocor for conducting the study. Zhong, de Boer, Uhlar, Marshall, Lang, Hait, and Ho were employees of Centocor during the conduct of the study.
Figures


References
-
- Albelda SM, Mette SA, Elder DE, Stewart R, Damjanovich L, Herlyn M, Buck CA (1990) Integrin distribution in malignant melanoma: association of the beta 3 subunit with tumor progression. Cancer Res 50: 6757–6764 - PubMed
-
- Atkins MB, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, Fleming ID, Gershenwald JE, Houghton Jr A, Kirkwood JM, McMasters KM, Mihm MF, Morton DL, Reintgen DS, Ross MI, Sober A, Thompson JA, Thompson JF (2002) Interleukin-2: clinical applications. Semin Oncol 29: 12–17
-
- Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, Fleming ID, Gershenwald JE, Houghton Jr A, Kirkwood JM, McMasters KM, Mihm MF, Morton DL, Reintgen DS, Ross MI, Sober A, Thompson JA, Thompson JF (2002) Melanoma of the skin. In AJCC Cancer Staging Manual, Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, Morrow M (eds) 6th edn, pp 209–220. Springer-Verlag: New York
-
- Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, Fleming ID, Gershenwald JE, Houghton Jr A, Kirkwood JM, McMasters KM, Mihm MF, Morton DL, Reintgen DS, Ross MI, Sober A, Thompson JA, Thompson JF (2001) Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 19: 3635–3648 - PubMed
-
- Bedikian AY, Millward M, Pehamberger H, Conry R, Gore M, Trefzer U, Pavlick AC, DeConti R, Hersh EM, Hersey P, Kirkwood JM, Haluska FG (2006) Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J Clin Oncol 24: 4738–4745 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

