Gene gain and loss during evolution of obligate parasitism in the white rust pathogen of Arabidopsis thaliana
- PMID: 21750662
- PMCID: PMC3130010
- DOI: 10.1371/journal.pbio.1001094
Gene gain and loss during evolution of obligate parasitism in the white rust pathogen of Arabidopsis thaliana
Abstract
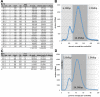
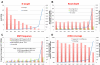
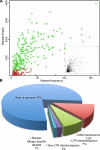
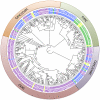
Biotrophic eukaryotic plant pathogens require a living host for their growth and form an intimate haustorial interface with parasitized cells. Evolution to biotrophy occurred independently in fungal rusts and powdery mildews, and in oomycete white rusts and downy mildews. Biotroph evolution and molecular mechanisms of biotrophy are poorly understood. It has been proposed, but not shown, that obligate biotrophy results from (i) reduced selection for maintenance of biosynthetic pathways and (ii) gain of mechanisms to evade host recognition or suppress host defence. Here we use Illumina sequencing to define the genome, transcriptome, and gene models for the obligate biotroph oomycete and Arabidopsis parasite, Albugo laibachii. A. laibachii is a member of the Chromalveolata, which incorporates Heterokonts (containing the oomycetes), Apicomplexa (which includes human parasites like Plasmodium falciparum and Toxoplasma gondii), and four other taxa. From comparisons with other oomycete plant pathogens and other chromalveolates, we reveal independent loss of molybdenum-cofactor-requiring enzymes in downy mildews, white rusts, and the malaria parasite P. falciparum. Biotrophy also requires "effectors" to suppress host defence; we reveal RXLR and Crinkler effectors shared with other oomycetes, and also discover and verify a novel class of effectors, the "CHXCs", by showing effector delivery and effector functionality. Our findings suggest that evolution to progressively more intimate association between host and parasite results in reduced selection for retention of certain biosynthetic pathways, and particularly reduced selection for retention of molybdopterin-requiring biosynthetic pathways. These mechanisms are not only relevant to plant pathogenic oomycetes but also to human pathogens within the Chromalveolata.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Comment in
-
Why biotrophs can't live alone.PLoS Biol. 2011 Jul;9(7):e1001097. doi: 10.1371/journal.pbio.1001097. Epub 2011 Jul 5. PLoS Biol. 2011. PMID: 21750665 Free PMC article. No abstract available.
References
-
- Yarwood C. E. Obligate parasitism. Ann Rev Plant Physiol. 1956;7:115–142.
-
- Goker M, Voglmayr H, Riethmuller A, Oberwinkler F. How do obligate parasites evolve? A multi-gene phylogenetic analysis of downy mildews. Fungal Genet Biol. 2007;44:105–122. - PubMed
-
- Spanu P. D, Abbott J. C, Amselem J, Burgis T. A, Soanes D. M, et al. Genome expansion and gene loss in powdery mildew fungi reveal tradeoffs in extreme parasitism. Science. 2010;330:1543–1546. - PubMed
-
- Friml J, Wisniewska J, Benkova E, Mendgen K, Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature. 2002;415:806–809. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

