Fast two-layer two-photon imaging of neuronal cell populations using an electrically tunable lens
- PMID: 21750778
- PMCID: PMC3130587
- DOI: 10.1364/BOE.2.002035
Fast two-layer two-photon imaging of neuronal cell populations using an electrically tunable lens
Abstract
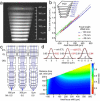
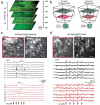
Functional two-photon Ca(2+)-imaging is a versatile tool to study the dynamics of neuronal populations in brain slices and living animals. However, population imaging is typically restricted to a single two-dimensional image plane. By introducing an electrically tunable lens into the excitation path of a two-photon microscope we were able to realize fast axial focus shifts within 15 ms. The maximum axial scan range was 0.7 mm employing a 40x NA0.8 water immersion objective, plenty for typically required ranges of 0.2-0.3 mm. By combining the axial scanning method with 2D acousto-optic frame scanning and random-access scanning, we measured neuronal population activity of about 40 neurons across two imaging planes separated by 40 μm and achieved scan rates up to 20-30 Hz. The method presented is easily applicable and allows upgrading of existing two-photon microscopes for fast 3D scanning.
Keywords: (170.0180) Microscopy; (170.2520) Fluorescence microscopy; (180.2520) Fluorescence microscopy; (180.4315) Nonlinear microscopy; (180.6900) Three-dimensional microscopy.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
