An evaluation of peptide-bond isosteres
- PMID: 21751326
- PMCID: PMC3253576
- DOI: 10.1002/cbic.201100272
An evaluation of peptide-bond isosteres
Abstract
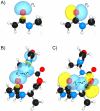
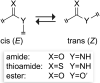
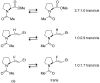
Peptide-bond isosteres can enable a deep interrogation of the structure and function of a peptide or protein by amplifying or attenuating particular chemical properties. In this Minireview, the electronic, structural, and conformational attributes of four such isosteres-thioamides, esters, alkenes, and fluoroalkenes-are examined in detail. In particular, the ability of these isosteres to partake in noncovalent interactions is compared with that of the peptide bond. The consequential perturbations provide a useful tool for chemical biologists to reveal new structure-function relationships, and to endow peptides and proteins with desirable attributes.
Copyright © 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



References
-
- Vickery HB, Schmidt CLA. Chem. Rev. 1931;9:169–318.
-
- Hofmeister F. Ergebnisse der Physiologie. 1902;1:759–802.
- Teich M, Needham DM. A Documentary History of Biochemistry. Fairleigh Dickinson University Press; Rutherford, NJ: 1992. pp. 1770–1940.
-
- Fischer E. Untersuchungen über Aminosäuren, Polypeptide und Proteïne (1899–1906) Verlag von Julius Springer; Berlin: 1906.
-
- Radzicka A, Wolfenden R. J. Am. Chem. Soc. 1996;118:6105–6109.
-
- Pauling L. The Nature of the Chemical Bond. 3rd ed. Cornell University Press; Ithaca, NY: 1960.
- Wiberg KB, Laidig KE. J. Am. Chem. Soc. 1987;109:5935–5943.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

