Catechol polymers for pH-responsive, targeted drug delivery to cancer cells
- PMID: 21751810
- PMCID: PMC3149454
- DOI: 10.1021/ja203077x
Catechol polymers for pH-responsive, targeted drug delivery to cancer cells
Abstract
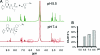
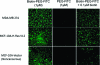
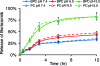
A novel cell-targeting, pH-sensitive polymeric carrier was employed in this study for delivery of the anticancer drug bortezomib (BTZ) to cancer cells. Our strategy is based on facile conjugation of BTZ to catechol-containing polymeric carriers that are designed to be taken up selectively by cancer cells through cell surface receptor-mediated mechanisms. The polymer used as a building block in this study was poly(ethylene glycol), which was chosen for its ability to reduce nonspecific interactions with proteins and cells. The catechol moiety was exploited for its ability to bind and release borate-containing therapeutics such as BTZ in a pH-dependent manner. In acidic environments, such as in cancer tissue or the subcellular endosome, BTZ dissociates from the polymer-bound catechol groups to liberate the free drug, which inhibits proteasome function. A cancer-cell-targeting ligand, biotin, was presented on the polymer carriers to facilitate targeted entry of drug-loaded polymer carriers into cancer cells. Our study demonstrated that the cancer-targeting drug-polymer conjugates dramatically enhanced cellular uptake, proteasome inhibition, and cytotoxicity toward breast carcinoma cells in comparison with nontargeting drug-polymer conjugates. The pH-sensitive catechol-boronate binding mechanism provides a chemoselective approach for controlling the release of BTZ in targeted cancer cells, establishing a concept that may be applied in the future toward other boronic acid-containing therapeutics to treat a broad range of diseases.
© 2011 American Chemical Society
Figures



References
-
- Kwon G. S.; Kataoka K. Adv. Drug Delivery Rev. 1995, 16, 295–309.
-
- Duncan R. Nat. Rev. Cancer 2006, 6, 688–701. - PubMed
-
- Rapoport N. Prog. Polym. Sci. 2007, 32, 962–990.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

