Encapsulation of exenatide in poly-(D,L-lactide-co-glycolide) microspheres produced an investigational long-acting once-weekly formulation for type 2 diabetes
- PMID: 21751887
- PMCID: PMC3202891
- DOI: 10.1089/dia.2011.0050
Encapsulation of exenatide in poly-(D,L-lactide-co-glycolide) microspheres produced an investigational long-acting once-weekly formulation for type 2 diabetes
Abstract
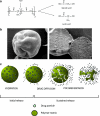
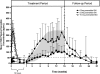
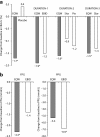
Exenatide once-weekly (EQW [2 mg s.c.]) is under development as monotherapy as an adjunct to diet and exercise or as a combination therapy with an oral antidiabetes drug(s) in adults with type 2 diabetes. This long-acting formulation contains the active ingredient of the original exenatide twice-daily (EBID) formulation encapsulated in 0.06-mm-diameter microspheres of medical-grade poly-(D,L-lactide-co-glycolide) (PLG). After mechanical suspension and subcutaneous injection by the patient, EQW microspheres hydrate in situ and adhere to one another to form an amalgam. A small amount of loosely bound surface exenatide, typically less than 1%, releases in the first few hours, whereas drug located in deeper interstices diffuses out more slowly (time to maximum, ~2 weeks). Fully encapsulated exenatide (i.e., drug initially inaccessible to diffusion) releases over a still longer period (time to maximum, ~7 weeks) as the PLG matrix hydrolyzes into lactic acid and glycolic acid, which are subsequently eliminated as carbon dioxide and water. For EQW, plasma exenatide concentrations reach the therapeutic range by 2 weeks and steady state by 6-7 weeks. This gradual approach to steady state seems to improve tolerability, as nausea is less frequent with EQW than EBID. EQW administrations may be associated with palpable skin nodules that generally resolve without further medical intervention. In comparative trials, EQW improved hemoglobin A1c more than EBID, sitagliptin, pioglitazone, or insulin glargine and reduced fasting plasma glucose more than EBID. Weight loss due to EQW or EBID was similar. EQW is the first glucose-lowering agent that is administered once weekly.
Figures



References
-
- Lovshin JA. Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:262–269. - PubMed
-
- Norris SL. Lee N. Thakurta S. Chan BK. Exenatide efficacy and safety: a systematic review. Diabet Med. 2009;26:837–846. - PubMed
-
- Gentilella R. Bianchi C. Rossi A. Rotella CM. Exenatide: a review from pharmacology to clinical practice. Diabetes Obes Metab. 2009;11:544–556. - PubMed
-
- Montanya E. Sesti G. A review of efficacy and safety data regarding the use of liraglutide, a once-daily human glucagon-like peptide 1 analogue, in the treatment of type 2 diabetes mellitus. Clin Ther. 2009;31:2472–2488. - PubMed
-
- Drab SR. Clinical studies of liraglutide, a novel, once-daily human glucagon-like peptide-1 analog for improved management of type 2 diabetes mellitus. Pharmacotherapy. 2009;29:43S–54S. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
