Unprecedented C-2 arylation of indole with diazonium salts: Syntheses of 2,3-disubstituted indoles and their antimicrobial activity
- PMID: 21752646
- PMCID: PMC3233240
- DOI: 10.1016/j.bmcl.2011.06.081
Unprecedented C-2 arylation of indole with diazonium salts: Syntheses of 2,3-disubstituted indoles and their antimicrobial activity
Abstract
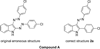
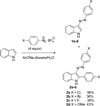
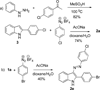
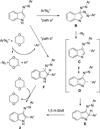
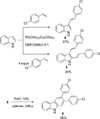
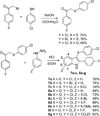
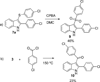
A novel reaction of indole with aryldiazonium salts leading to the formation of 2-aryl-3-(arylazo)indoles was discovered. The products were found to possess potent anti-MRSA and anti-LLVRE activities. The SAR studies indicate that the potentially metabolically labile azo functionality can be replaced with ether oxygen and thioether sulfur atoms without any loss of activity.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
References
-
- Pichereau S, Rose WE. Expert Opin. Pharmacother. 2010;11:3009. - PubMed
-
- Tacconelli E, Cataldo MA. Int. J. Antimicrob. Agents. 2008;31:99. - PubMed
-
- Kobayashi Y, Ichioka M, Hirose T, Nagai K, Matsumoto A, Matsui H, Hanaki H, Masuma R, Takahashi Y, Omura S, Sunazuka T. Bioorg. Med. Chem. Lett. 2010;20:6116. - PubMed
-
- Hossion AML, Otsuka N, Kandahary RK, Tsuchiya T, Ogawa W, Iwado A, Zamami Y, Sasaki K. Bioorg. Med. Chem. Lett. 2010;20:5349. - PubMed
-
- Hu L, Kully ML, Boykin DW, Abood N. Bioorg. Med. Chem. Lett. 2009;19:4626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous