The use of trehalose in the preparation of specimens for molecular electron microscopy
- PMID: 21752659
- PMCID: PMC3156378
- DOI: 10.1016/j.micron.2011.06.005
The use of trehalose in the preparation of specimens for molecular electron microscopy
Abstract
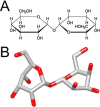
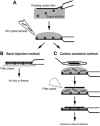
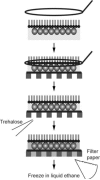
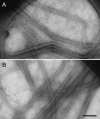
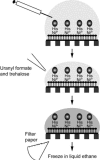
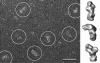
Biological specimens have to be prepared for imaging in the electron microscope in a way that preserves their native structure. Two-dimensional (2D) protein crystals to be analyzed by electron crystallography are best preserved by sugar embedding. One of the sugars often used to embed 2D crystals is trehalose, a disaccharide used by many organisms for protection against stress conditions. Sugars such as trehalose can also be added to negative staining solutions used to prepare proteins and macromolecular complexes for structural studies by single-particle electron microscopy (EM). In this review, we describe trehalose and its characteristics that make it so well suited for preparation of EM specimens and we review specimen preparation methods with a focus on the use of trehalose.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures


References
-
- Abeyrathne PD, Chami M, Pantelic RS, Goldie KN, Stahlberg H. Preparation of 2D crystals of membrane proteins for high-resolution electron crystallography data collection. Methods Enzymol. 2010;481:25–43. - PubMed
-
- Adrian M, Dubochet J, Lepault J, McDowall AW. Cryo-electron microscopy of viruses. Nature. 1984;308:32–36. - PubMed
-
- Adrian M, Dubochet J, Fuller SD, Harris JR. Cryo-negative staining. Micron. 1998;29:145–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

